Key Points
UFH, LMWH, and NAC restored angiogenesis in decorin-reduced endothelial cells.
NAC treatment was similar to, or better than, UFH or LMWH at improving endothelial angiogenesis without increasing anticoagulant activity.
Abstract
Pregnancies affected by preeclampsia (PE) or fetal growth restriction (FGR) display increases in thrombin generation and reductions in angiogenesis and cell growth. There is significant interest in the potential for low molecular weight heparins (LMWHs) to reduce the recurrence of PE and FGR. However, LMWH is associated with an increased risk of bleeding. Therefore, it is of vital importance to determine the exact molecular function of heparins in pregnancy if they are used as therapy for pregnant women. We aimed to determine this using our model for PE/FGR in microvascular endothelial cells. The expression of decorin, a proteoglycan, was reduced to mimic PE/FGR in these cells compared with controls. Four concentrations of unfractionated heparin (UFH), LMWH, and nonanticoagulant heparin (NAC) were added to determine the effect on thrombin generation, angiogenesis, and cell growth. Treatment with UFH and LMWH reduced thrombin generation and restored angiogenesis but decreased cell growth. Treatment with NAC did not affect thrombin generation, restored angiogenesis, and showed a trend toward cell growth. In conclusion, treatment with NAC produced the same, if not better, results as treatment with UFH or LMWH, without the same impact on coagulation. Therefore, NAC could potentially be a better therapeutic option for prevention of PE/FGR in high-risk women, without the risk of the adverse effects of traditional anticoagulants.
Introduction
A successful pregnancy requires the establishment and proper development of an adequate placental circulation. Placenta-mediated pregnancy complications include preeclampsia (PE), fetal growth restriction (FGR), and late pregnancy loss. It has been documented that these complications are associated with abnormal placental development, with underdeveloped placental vasculature or placental inflammation.1,2 In addition, normal pregnancy is considered a hypercoagulable state and a period of increased risk of thrombotic complications. This is accompanied by all the elements of Virchow’s triad: hypercoagulability, venous stasis, and vascular damage.3 Therefore, thrombosis in the placental bed is also at least partially responsible for placenta-mediated complications.4-6
Apart from the devastating effects of these pregnancy complications on both maternal and fetal health, the risk of recurrent placenta-mediated pregnancy complications is substantial. For example, women affected by prior severe PE have a 25% to 65% risk of recurrent PE, a 3% risk of placental abruption, and a 10% risk of small-for-gestation-age babies.6,7 There are currently no highly effective preventive strategies that can be used for the prevention of these complications. Aspirin has been shown to offer a small relative risk reduction in patients with prior PE and small-for-gestation-age babies; however, meta-analyses suggest it is only effective if started within the first 16 weeks of pregnancy.8,9
In recent years, anticoagulants such as heparin have been used increasingly to try to prevent recurrent pregnancy complications by reduction of thrombosis or utilization of the anti-inflammatory and proangiogenic functions of heparin. Although several randomized controlled trials have been undertaken to determine whether low molecular weight heparin (LMWH) can prevent recurrent pregnancy complications, the results have not been universal, and the studies have been underpowered and had inconsistent patient criteria.10-12 Therefore, although it seems that LMWH could be a promising therapeutic treatment for recurrent pregnancy complications, there is still insufficient evidence proving the efficacy and impact of LMWH treatment during pregnancy. Furthermore, adoption of this intervention without sufficient information of its potential benefits and harms could expose women to the risk of undesirable and potentially fatal adverse effects, such as major bleeding, heparin-induced thrombocytopenia, osteoporotic fractures, and withholding of epidural analgesia.13,14 Therapy is also associated with regular injections and substantial costs.
Heparin is a glycosaminoglycan composed of chains of alternating residues of d-glucosamine and uronic acid. Its unique pentasaccharide structure has a high binding affinity to antithrombin,15 and this mediates the majority of the anticoagulant effect. Unfractionated heparin (UFH) has been used for many indications during pregnancy. It is a large molecule that does not cross the placenta and therefore does not cause teratogenic effects, unlike coumarin (Warfarin). The main adverse effects of UFH are inconvenience, with a minimum of twice daily injections, and the potential for osteoporosis and heparin-induced thrombocytopenia. LMWHs, in contrast, have become the preferred anticoagulant because they are equivalent or superior to UFH in efficacy and safety in the treatment of thrombotic problems outside of pregnancy.16-19 Several studies have suggested the safety and efficacy of LMWH during pregnancy.13,20,21 In addition, the risk of adverse heparin-induced thrombocytopenia and osteoporosis after LMWH treatment is greatly reduced compared with UFH.20-25
In our previous work, we showed that the messenger RNA and protein expression of the proteoglycan decorin (DCN) was reduced in PE- and FGR-affected placentae compared with controls.26-28 Furthermore, we also showed that downregulation of DCN in human microvascular endothelial cells resulted in decreased cell growth and proliferation, decreased network formation (angiogenesis), and a modest increase in thrombin generation.26 We also demonstrated that DCN was downregulated in the first trimester in women who went on to develop growth-restricted infants, suggesting that there is both a temporal relationship and biologic plausibility for the association between reduced DCN expression and subsequent development of PE/FGR.29 The results from these studies have led to the opportunity to investigate whether the addition of heparin to these DCN-reduced microvascular endothelial cells may result in a reversal of adverse cell growth and angiogenesis, as well as an expected anticoagulation effect. In addition, we also aimed to investigate the effect of adding a nonanticoagulant (NAC), de-N-desulfated heparin, to the DCN-reduced cells to determine whether this heparin could also reverse the decrease in cell growth and angiogenesis observed, but without the detrimental anticoagulant effects during pregnancy. We used a wide range of heparin concentrations from the therapeutic doses according to the American College of Chest Physicians guidelines,30 with one concentration above and below these values. This study will be the first to determine the effect of heparin (UFH, LMWH, and NAC) on cultured microvascular endothelial cells and determine whether this addition may help to reverse some of the adverse effects of DCN downregulation.
Methods
Cell lines
The telomerase-immortalized human microvascular endothelial cells from neonatal foreskin (TIME) were purchased from American Type Culture Collection (CRL-4025; Manassas, VA). TIME cells were cultured in Microvascular Endothelial Cell Growth Medium-2 (EGM-2 MV; Single Quot Kit; catalog number CC-4147; Lonza/Clonetics, Mt Waverley, VIC, Australia) containing 10% fetal bovine serum (Murdoch Childrens Research Institute Tissue Culture Supplies, Parkville, VIC, Australia) at 20% oxygen culture conditions. These cells have been shown to represent the functional and morphological characteristics of human placental endothelial cells.31
Reduction of DCN expression by siRNA
Four independent DCN small interference (siRNA) oligonucleotides were obtained as 4-For-Silencing siRNA Duplexes (Qiagen, Chadstone, VIC, Australia). The DCN siRNAs showed no significant DNA sequence similarity to other genes in GenBank complementary DNA databases (data not shown).26
TIME cells were grown in EGM-2 MV and transfected with DCN siRNAs using HiPerfect transfection reagent (Qiagen). Negative control (NC) siRNA consisted of a pool of enzyme-generated siRNA oligonucleotides that were not specific for any known human genes (AllStars Negative siRNA; Qiagen).26 The Mock control represented the cells in media only. The efficacies of the 4 siRNAs in downregulating DCN, and the subsequent selection of the 2 best siRNA as DCNS2 and DCNS3, are depicted in our previous published work.26
Heparins and concentrations used
The following heparin concentrations were used: UFH, heparin sodium 5000 IU/mL (Pfizer, West Ryde, NSW, Australia); LMWH, dalteparin 10 000 IU/mL; and NAC, de-N-sulfated heparin sodium salt 5 mg, purchased from Sigma (Castle Hill, NSW, Australia) as prepared from porcine mucosal heparin by a modification of the method to render it completely without anticoagulant activity.32
The actual concentration of heparins used was adjusted for tissue culture experiments to correspond to those used in an adult at prophylactic and therapeutic ranges. Therefore, because the therapeutic range of UFH in adults is usually aimed at 0.35 to 0.7 IU/mL as measured by anti-Xa activity, we used UFH at 0.15, 0.35, 0.5, and 1.0 IU/mL to cover this range. Similarly, the therapeutic range of LMWH in adults is between 0.5 and 1.0 IU/mL, also measured by anti-Xa activity; our LMWH concentrations were 0.15, 0.5, 0.7, and 1.2 IU/mL. Because NAC has not been used therapeutically, we tried to use concentrations similar to those used for UFH and LMWH as milligrams per milliliter. The concentrations we used for NAC were 0.010, 0.026, 0.040, and 0.073 mg/mL.
Stock solutions of UFH, LMWH, and NAC were made up in complete EGM-2 MV media and used throughout all experiments that contained the heparins.
TIME cell growth using the xCELLigence system with addition of UFH, LMWH, and NAC
TIME cell growth was assessed using the xCELLigence SP real-time system (Roche Diagnostics, Melbourne, VIC, Australia) according to the manufacturer’s instructions.26 Briefly, cells were prepared and added to the E-Plate 96 at a density of 5000 cells per well and transfected with DCN siRNAs or Mock/NC for 48 hours in 4 concentrations of each of UFH, LMWH, or NAC (Roche Diagnostics). The xCELLigence system recorded the background electrical impedance for 72 hours. The cell index was calculated using the RTCA-integrated software (version 1.2; John Morris Scientific, Melbourne, VIC, Australia), and the data were analyzed using GraphPad Prism 5 (GraphPad Software, San Diego, CA).
TIME cell network formation assays with addition of UFH, LMWH, and NAC
TIME cell network formation was assessed using the µ-Slide Angiogenesis system (IBIDI, Hallam, VIC, Australia).26 Briefly, TIME cells were transfected with DCN siRNA or Mock/NC in 24-well plates with 4 concentrations of each of UFH, LMWH, or NAC and incubated for 24 hours. After the treatment, the cells were dissociated using enzyme-free cell dissociation solution (Millipore, Billerica, MA). The µ-Slide Angiogenesis wells were coated with 10 µl of neat Growth-Factor Reduced Matrigel (BD, Scoresby, VIC, Australia) and allowed to polymerize for 1 hour at room temperature. The cells were seeded into the wells of the slide at a density of 8000 cells per well and returned to the incubator for an additional 24 hours, for a total siRNA/Mock/NC plus heparin incubation time of 48 hours. The media was then removed, stained with calcein-AM (Millipore), and visualized under the Olympus BX53 fluorescence microscope (Olympus, Tokyo, Japan). Photomicrographs at a magnification of ×10 of entire wells were taken in triplicate, and the branching points between the TIME cells were counted by Wimasis image analysis (Munich, Germany).
Thrombin-generation assays with addition of UFH, LMWH, and NAC
TIME cells were plated into 96-well plates at a density of 5000 cells per well and transfected with DCN siRNAs or Mock/NC with 4 concentrations of each of UFH, LMWH, or NAC for 48 hours. Lyophilized Coag-Norm plasma (Diagnostica Stago, Doncaster, VIC, Australia) was obtained and resuspended as per manufacturer’s instructions. This was then spiked with 4 concentrations of UFH, LMWH, or NAC. Measurement of endogenous thrombin potential (ETP) by calibrated automated thrombogram (CAT; Thrombinoscope; Diagnostica Stago) was performed according to the manufacturer’s instructions.26 All experiments were conducted in triplicate wells. The ETP represents the total enzymatic activity performed by thrombin, which is considered the most predictive parameter of bleeding/thrombosis risk.33,34 The ETP (nanomolar per minute) was calculated using the Thrombinoscope software (version 3.0.0.29; Diagnostica Stago) and represented as the area under the thrombin-generation curve.
Data analysis
All data in this study are described as mean ± standard error of the mean and were analyzed by the GraphPad Prism 6 statistical software (GraphPad Software). One-way analysis of variance (ANOVA) was used to assess the differences in DCN function between siRNA-treated and Mock/NC groups with or without added heparins. A probability value of < .05 was considered statistically significant.
Results
Thrombin-generation potential
Treatment with all 4 concentrations of UFH and LMWH decreased thrombin-generation levels of control and DCN-reduced TIME cells, with the exception of NAC, which had no statistically significant effects on thrombin generation at lower concentrations of NAC but a modest effect at the highest dose of NAC.
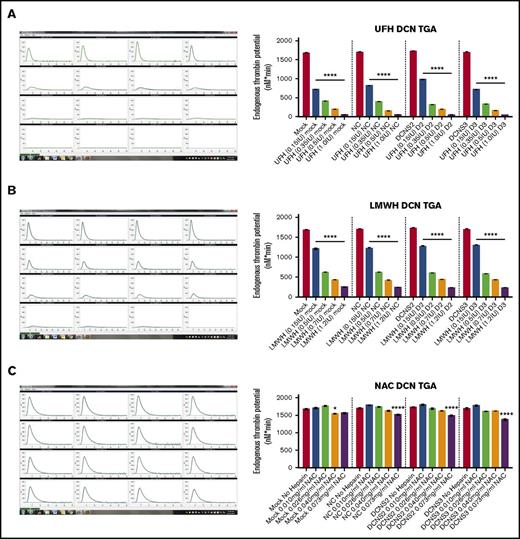
In Figure 1, the panels on the left column are representative images of the output from the CAT machine showing the thrombin-generation curve and how the ETP, or area under the curve, is calculated. The y-axis is in nanomolars and the x-axis is the time in minutes. Using the ETP calculated by the CAT program, treatment with all 4 concentrations of UFH or LMWH resulted in a significant dose-dependent decrease in the thrombin-generation potential of DCN-reduced TIME cells compared within each treatment group (P < .00001; n = 9; 1-way ANOVA; Figure 1A-B). However, treatment with NAC did not result in a decrease in ETP when compared within each individual treatment group (P > .05; n = 9; 1-way ANOVA), except at the higher concentrations of 0.6 IU (Mock vs Mock 0.6 IU: P < .01; n = 9; 1-way ANOVA) or 1.2 IU (NC vs NC 1.2 IU, D2 vs D2 1.2 IU, and D3 vs D3 1.2 IU: P < .00001; n = 9; 1-way ANOVA; Figure 1C).
Treatment with all 4 concentrations of UFH and LMWH decreased thrombin generation levels of control and DCN-reduced TIME cells, with the exception of NAC. The ETP of the TIME cells after reduction of DCN and incubated with heparins was determined using the CAT system. Representative images from the CAT output are also shown for each heparin used, where the y-axis represents the ETP in nanomolars and the x-axis shows the time in minutes. (A) Treatment without heparin and treatment with all 4 concentrations of UFH (A), LMWH (B), and NAC (C) in a dose-dependent manner. *P < .01, ****P < .00001 (both significant); n = 9; 1-way ANOVA. The y-axis represents the ETP (nanomolars per minute).
Treatment with all 4 concentrations of UFH and LMWH decreased thrombin generation levels of control and DCN-reduced TIME cells, with the exception of NAC. The ETP of the TIME cells after reduction of DCN and incubated with heparins was determined using the CAT system. Representative images from the CAT output are also shown for each heparin used, where the y-axis represents the ETP in nanomolars and the x-axis shows the time in minutes. (A) Treatment without heparin and treatment with all 4 concentrations of UFH (A), LMWH (B), and NAC (C) in a dose-dependent manner. *P < .01, ****P < .00001 (both significant); n = 9; 1-way ANOVA. The y-axis represents the ETP (nanomolars per minute).
Network formation
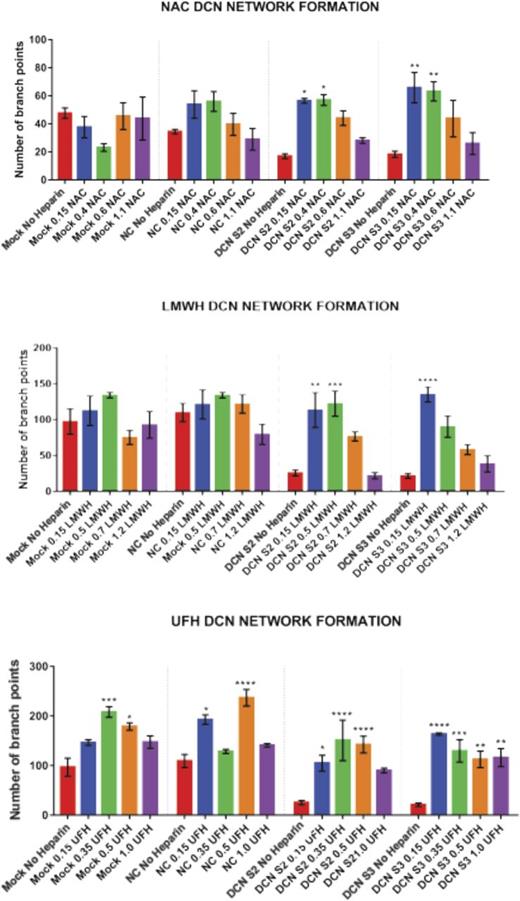
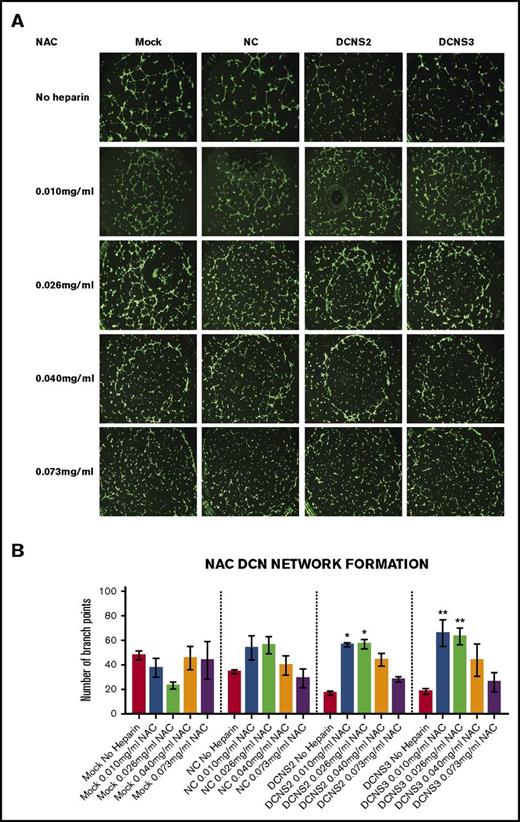
Treatment with all 4 concentrations of UFH, LMWH, or NAC restored the network-formation abilities of DCN-reduced TIME cells. Figures 2-4 show representative images of the network-formation potential of TIME cells, quantitated by the number of branch points.
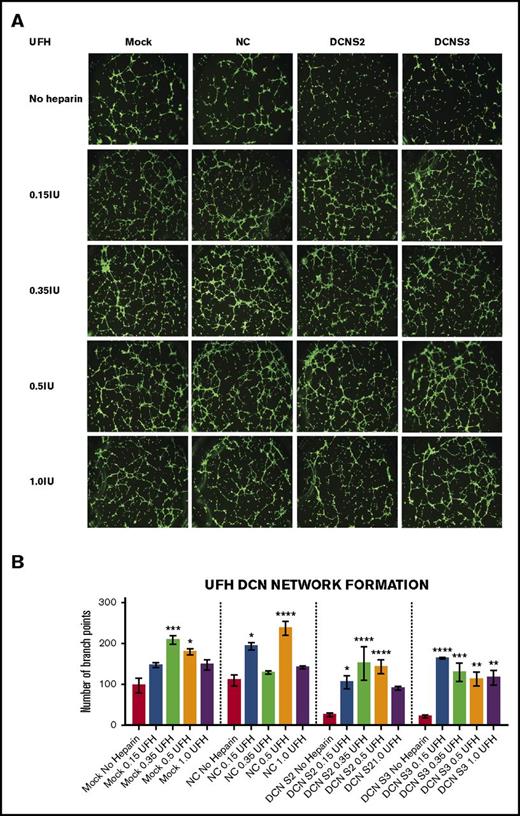
Treatment with all 4 concentrations of UFH restored the network-formation abilities of DCN-reduced TIME cells. The ability of TIME cells to form networks after DCN siRNA or Mock/NC was determined using the μ-Slide Angiogenesis system by IBIDI. (A) Representative image of the networks stained with calcein-AM is shown for each concentration. Images were acquired using the Olympus BX53 fluorescence microscope and represent 10× magnification to obtain the whole field. (B) The network-formation potential of TIME cells after treatment with 4 concentrations of UFH calculated by the Wimasis software. *P < .05, **P < .001, ***P < .0001, ****P < .00001 (all significant); n = 9; 1-way ANOVA. The y-axis represents the number of branch points.
Treatment with all 4 concentrations of UFH restored the network-formation abilities of DCN-reduced TIME cells. The ability of TIME cells to form networks after DCN siRNA or Mock/NC was determined using the μ-Slide Angiogenesis system by IBIDI. (A) Representative image of the networks stained with calcein-AM is shown for each concentration. Images were acquired using the Olympus BX53 fluorescence microscope and represent 10× magnification to obtain the whole field. (B) The network-formation potential of TIME cells after treatment with 4 concentrations of UFH calculated by the Wimasis software. *P < .05, **P < .001, ***P < .0001, ****P < .00001 (all significant); n = 9; 1-way ANOVA. The y-axis represents the number of branch points.
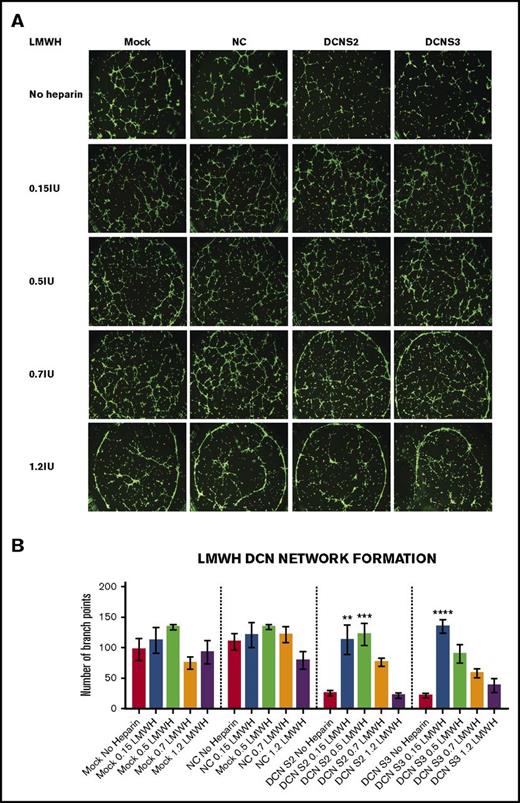
Treatment with all 4 concentrations of LMWH restored the network-formation abilities of DCN-reduced TIME cells. (A) Representative image of the networks stained with calcein-AM is shown for each concentration. Images were acquired using the Olympus BX53 fluorescence microscope and represent 10× magnification to obtain the whole field. (B) The network-formation potential of TIME cells after treatment with LMWH calculated by the Wimasis software. **P < .001, ***P < .0001, ****P < .00001 (all significant); n = 9; 1-way ANOVA. The y-axis represents the number of branch points.
Treatment with all 4 concentrations of LMWH restored the network-formation abilities of DCN-reduced TIME cells. (A) Representative image of the networks stained with calcein-AM is shown for each concentration. Images were acquired using the Olympus BX53 fluorescence microscope and represent 10× magnification to obtain the whole field. (B) The network-formation potential of TIME cells after treatment with LMWH calculated by the Wimasis software. **P < .001, ***P < .0001, ****P < .00001 (all significant); n = 9; 1-way ANOVA. The y-axis represents the number of branch points.
Treatment with all 4 concentrations of NAC restored the network-formation abilities of DCN-reduced TIME cells. (A) Representative image of the networks stained with calcein-AM is shown for each concentration. Images were acquired using the Olympus BX53 fluorescence microscope and represent 10× magnification to obtain the whole field. (B) Network formation of TIME cells after treatment with NAC calculated by the Wimasis software. *P < .05, **P < .001 (both significant); n = 9; 1-way ANOVA. The y-axis represents the number of branch points.
Treatment with all 4 concentrations of NAC restored the network-formation abilities of DCN-reduced TIME cells. (A) Representative image of the networks stained with calcein-AM is shown for each concentration. Images were acquired using the Olympus BX53 fluorescence microscope and represent 10× magnification to obtain the whole field. (B) Network formation of TIME cells after treatment with NAC calculated by the Wimasis software. *P < .05, **P < .001 (both significant); n = 9; 1-way ANOVA. The y-axis represents the number of branch points.
UFH.
Treatment with the midconcentrations of UFH 0.35 and 0.5 IU, but not the highest, resulted in a significant increase in branch points in Mock (0.35 IU: P < .0001; 0.5 IU: P < .05; n = 9; 1-way ANOVA) or NC (0.15 IU: P < .05; 0.5 IU: P < .00001; n = 9; 1-way ANOVA). Importantly, treatment with almost all 4 concentrations of UFH resulted in an increase in branch points in DCN-reduced cells back to non–heparin-treated Mock/NC levels (P < .05 to P < .00001; n = 9; 1-way ANOVA; Figure 2A-B).
LMWH
Unlike UFH, treatment with the low concentrations to midconcentrations of LMWH, but not the higher concentrations, significantly increased branch points back to non–heparin-treated Mock/NC levels in DCN-reduced cells (P < .001 to P < .00001; n = 9; 1-way ANOVA), without affecting Mock- or NC-treated cells (Figure 3A-B).
NAC
Similar to LMWH, only treatment with the low concentrations to midconcentrations of NAC significantly restored branch points to non–heparin-treated Mock/NC levels in DCN-reduced cells (P < .05 to P < .001; n = 9; 1-way ANOVA), without affecting heparin-treated Mock or NC cells (Figure 4A-B).
Cell growth
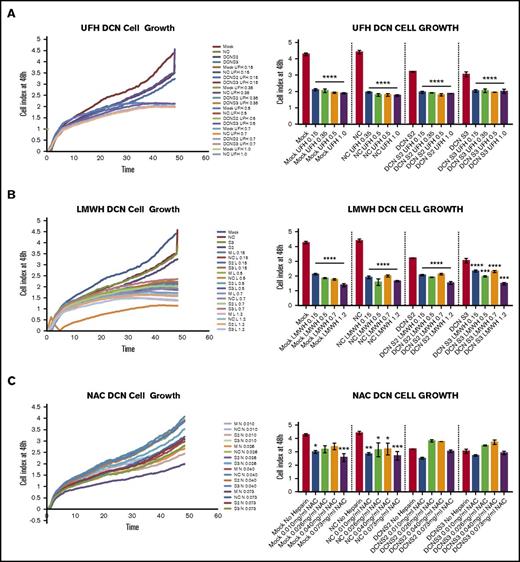
Treatment with all 4 concentrations of UFH or LMWH, but not NAC, reduced cell growth of DCN-reduced TIME cells at 48 hours, as shown in Figure 5. Graphical representation of cell index from time point 0 to 48 hours is also shown.
Treatment with all 4 concentrations of UFH or LMWH, but not NAC, reduced cell growth of DCN-reduced TIME cells. The effect on TIME-cell proliferation after 48 hours of incubation with DCN siRNA or Mock/NC incubated in 4 concentrations of different heparins was determined using the xCELLigence system. Representative images from the xCELLigence output are also shown for each heparin used, where the y-axis represents the cell index and the x-axis shows the time in minutes. (A) A representative graph showing the cell index of TIME cells treated with DCN siRNA or Mock/NC incubated with 0.15, 0.35, 0.5, and 1.0 IU of UFH over 48 hours in culture. ****P < .00001 (significant); n = 9; 1-way ANOVA. The y-axis represents the cell index at 48 hours. (B) The results after incubation with 0.15, 0.5, 0.7, and 1.2 IU of LMWH. ***P < .0001, ****P < .00001 (both significant); n = 9; 1-way ANOVA. The y-axis represents the cell index at 48 hours. (C) Treatment with 0.15, 0.4, 0.6, and 1.1 IU of NAC and cell growth in Mock/NC and DCN-reduced cells. *P < .05, **P < .001, ***P < .0001 (all significant); n = 9; 1-way ANOVA. The y-axis represents the cell index at 48 hours.
Treatment with all 4 concentrations of UFH or LMWH, but not NAC, reduced cell growth of DCN-reduced TIME cells. The effect on TIME-cell proliferation after 48 hours of incubation with DCN siRNA or Mock/NC incubated in 4 concentrations of different heparins was determined using the xCELLigence system. Representative images from the xCELLigence output are also shown for each heparin used, where the y-axis represents the cell index and the x-axis shows the time in minutes. (A) A representative graph showing the cell index of TIME cells treated with DCN siRNA or Mock/NC incubated with 0.15, 0.35, 0.5, and 1.0 IU of UFH over 48 hours in culture. ****P < .00001 (significant); n = 9; 1-way ANOVA. The y-axis represents the cell index at 48 hours. (B) The results after incubation with 0.15, 0.5, 0.7, and 1.2 IU of LMWH. ***P < .0001, ****P < .00001 (both significant); n = 9; 1-way ANOVA. The y-axis represents the cell index at 48 hours. (C) Treatment with 0.15, 0.4, 0.6, and 1.1 IU of NAC and cell growth in Mock/NC and DCN-reduced cells. *P < .05, **P < .001, ***P < .0001 (all significant); n = 9; 1-way ANOVA. The y-axis represents the cell index at 48 hours.
UFH.
Figure 5A is representative graph showing the cell index of TIME cells treated with DCN siRNA or Mock/NC incubated with 0.15, 0.35, 0.5, and 1.0 IU of UFH over 48 hours in culture. All 4 concentrations of UFH resulted in a significant decrease in cell growth in cells treated with Mock, NC, or DCN siRNA (P < .00001; n = 9; 1-way ANOVA).
LMWH.
The results after incubation with 0.15, 0.5, 0.7, and 1.2 IU of LMWH are shown in Figure 5B. Once again, all 4 concentrations of LMWH resulted in a significant decrease in cell growth in all cells treated (P < .0001 to P < .00001; n = 9; 1-way ANOVA).
NAC.
Treatment with 0.15, 0.4, 0.6, and 1.1 IU of NAC resulted in a significant decrease in cell growth in Mock- and NC-treated cells (P < .01; n = 9; 1-way ANOVA), but not in DCN-reduced cells, and did not restore cell growth to non–heparin-treated Mock/NC levels (P > .05 to P < .0001; n = 9; 1-way ANOVA; Figure 5C).
Discussion
Over the recent years, an increasing number of pregnant women have been given heparins to prevent a wide range of pregnancy-related complications, such as recurrent miscarriages,35 pregnancy-related thromboembolism,36 and recurrent PE/FGR. The most common heparin being used is LMWH, which has a lower risk of serious adverse effects when used over a short time during pregnancy. Heparins are complex macromolecules with diverse actions that extend beyond purely anticoagulation.37 Therefore, because pregnancy disorders such as PE and FGR are also complex pathologies, it makes sense to further elucidate the mechanisms by which heparins affect placental development.
Previous randomized controlled trials have reported a lack of efficacy of LMWH in the prevention of recurrent miscarriages in thrombophilia+ and thrombophilia− women.38-40 However, smaller trials have demonstrated that subcutaneous LMWH improved perinatal outcomes in thrombophilia− women with previous severe PE.41 In addition, over recent years, the activity and functional role of NAC heparins in different forms have been widely investigated, including in angiogenesis and pulmonary diseases.42-44 The anti-inflammatory role, via inhibition of leukocyte infiltration,45 and antimetastatic role in lung and colon46 and pancreatic cancer cells47 of these NAC heparins has also been established. However, their efficacy and safety in pregnancy have not been investigated.
In this study, we used UFH, LMWH, and NAC to determine whether addition of these agents would potentially reverse the effects of DCN downregulation, specifically network formation and cell growth.26 As expected, UFH and LMWH decreased the thrombin generation of the cells. In contrast, NAC had no effect on thrombin generation. Thrombin generation, with resultant placental infarcts, does not seem to be the main culprit in the development of pregnancy complications such as FGR and PE.48 Therefore, this result is significant because the use of NAC could potentially eliminate the clinical bleeding risks involved with using either UFH or LMWH in high-risk pregnant women. Moreover, the reduction of anticoagulant activity means that women are not prevented from accessing epidurals during labor and birth.
The addition of UFH, LMWH, and NAC to DCN-downregulated cells significantly reversed the reduction in network formation to at least Mock control or NC levels. However, UFH, the strongest anticoagulant used, also significantly increased network formation abilities of the cells in Mock controls and NCs, causing us to speculate that if this were used in women without pregnancy complications, the angiogenic potential of the microvascular endothelial cells in the placenta and potentially throughout the system would be increased. The effect and/or consequence of this increase have not been previously considered. However, the use of the lowest concentration of UFH, at 0.15 IU/mL, restored network formation to control level without affecting the controls themselves. Therefore, there is potential for the use of a lower concentration of UFH than what is currently used therapeutically. In contrast, this increase did not occur in controls treated with low doses to mid-doses of LMWH or NAC. The ability for LMWH to improve in vitro angiogenesis in serum of high-risk women to the equivalent of serum from low-risk women in human umbilical vein endothelial cells has been recently documented,49 substantiating the findings observed in the current experiments. In addition, the restoration of network formation occurred at the lowest doses of LMWH, therefore suggesting that at least in vitro, low doses have the potential to induce the beneficial effects in the placenta. Whether this translates to improvements in vivo at low doses is less certain, because various factors including maternal weight, body mass index, and renal function can alter the pharmacokinetics of heparins. The addition of low-dose NAC resulted in the same restorative effect in DCN-reduced cells as both UFH and LMWH. This is clinically significant because NAC could potentially be used in place of LMWH and still result in the same rescue effect. This could eliminate the anticoagulant risks posed by the heparins, leading to potentially increased therapeutic safety with the benefit of improving pregnancy outcomes.
Cell growth, or proliferation, is an integral part of any developed or developing organ system, including the placenta. DCN reduction resulted in a significant decrease in cell growth compared with controls.26 Treatment with all 4 doses of UFH or LMWH resulted in a significant further decrease in cell growth and thus did not rescue the effect of DCN reduction. Although there is limited information regarding the antiproliferative role of UFH or LMWH specifically in endothelial cells, it is well known that glycosaminoglycans play a role in endothelial cell function, where heparin and heparan sulfate can modulate the angiogenic and/or proliferative activities of growth factors such as fibroblast growth factor 250 by facilitating receptor interaction and activation.51,52 However, although heparins increased the angiogenic potential of TIME cells, the differential effects of fibroblast growth factor 2 signaling and function, including its separate role in proliferation, depend on specific structural variations (ie, variations in the molecular weight of heparin and heparan sulfate).53,54 Khorana et al55 reported an antiproliferative effect in cultured human umbilical vein endothelial cells of 94% and 58%, observed in 6kDa and 3kDa LMWH, respectively. In contrast, no data regarding the antiproliferative effect of UFH or LMWH in microvascular endothelial cells have been reported. The LMWH (Dalteparin) used in this study is a 3-6kDa heparin and is the same LMWH that is currently used during pregnancy. Therefore, a significant antiproliferative effect was observed even at the lowest dose of this LMWH, indicating that there may actually be some further detrimental potential of using this LMWH to prevent adverse pregnancy outcomes in terms of a reduction in endothelial cell growth. This is especially relevant if a decrease in cell growth is already a problem in cells affected by downregulation of DCN, as observed in first-trimester samples from women who went on to develop pregnancies complicated by FGR.29 In addition, women who do not have complicated pregnancies and are treated with the same LMWH may have detrimental effects in terms of endothelial cell growth. Alternatively, although a decrease in cell growth was also observed in control cells treated with doses of NAC, this decrease was not as significant as those observed for UFH and LMWH, and the DCN-reduced cells did not have an additional decrease in their cell-growth potential that was not already present in the non–heparin-treated cells. Therefore, if using our DCN-reduced cells as a model for PE/FGR, this treatment with NAC produced an overall reduction in cell growth but did not potentiate further reduction in the DCN-reduced cells. In other words, treatment with NAC may not exacerbate cell growth in women with PE and/or FGR compared with UFH or LMWH. Whether these in vitro effects translate into in vivo effects remains to be determined.
This study was based on our previous findings of the importance of DCN in the development of the human placenta and the potential contribution its downregulation has on the development of PE and/or FGR,26,29 together with the knowledge that heparins are currently used to prevent adverse pregnancy outcomes. We aimed to investigate the mechanism of heparin with respect to DCN downregulation in microvascular endothelial cells as our model for PE/FGR. In addition, the comparison between treatment with a NAC heparin and traditional anticoagulant heparins revealed that the NAC resulted in similar effects at a cellular level to LMWH or UFH, with additional benefits including improved cellular proliferation and reduced anticoagulant activity. This then raises the possibility that NAC could potentially be a better candidate for the prevention of PE/FGR without the risk of significant adverse effects associated with the currently used LMWH. As such, future research into the extended use of NAC and its interaction and implications in the control of cellular functions in other cell types and tissues may improve our understanding of this drug, as well as pave the way for potentially extending this into clinical trials.
Acknowledgments
This work was supported by National Health and Medical Research Council Project Grants, Australia, awarded to J.M.S. (Chief Investigator A) (Application 10042239).
Authorship
Contribution: A.K.L.C. contributed to the study concept, performed all experiments, data analysis, and interpretation, and wrote the manuscript; T.N.G. contributed to the study concept, performed some experiments, and performed critical review of manuscript draft; V.I. contributed to the study concept, performed some of the thrombin generation assays, and performed critical review of manuscript drafts and final approval of manuscript for submission; P.T.M., P.M., S.P.B., and J.M.W. contributed to the study concept and performed critical review of manuscript drafts and final approval of manuscript for submission; and J.M.S. participated in the study concept and design, obtained funding to undertake this work, and performed interpretation of data, critical review of manuscript drafts, and final approval of manuscript for submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Amy K. L. Chui, Department of Obstetrics and Gynaecology, The University of Melbourne, Sunshine Hospital, PO Box 294, 176 Furlong Rd, St Albans, VIC 3021, Australia; e-mail: akl.chui83@gmail.com.