Key Points
EPCR levels influence the hemostatic effect of rFVIIa in hemophilia therapy.
FVIIa binding to EPCR influences the hemostatic effect of FVIIa because of downregulation of protein C anticoagulation pathway.
Abstract
Recent studies established that clotting factor VIIa (FVIIa) binds endothelial cell protein C receptor (EPCR). It has been speculated that FVIIa interaction with EPCR might augment the hemostatic effect of recombinant FVIIa (rFVIIa) in therapeutic conditions. The present study is carried out to investigate the mechanism by which FVIIa interaction with EPCR contributes to the hemostatic effect of rFVIIa in hemophilia therapy. Active-site inhibited FVIIa, which is capable of binding to EPCR but has no ability to activate factor X, reduced the concentration of rFVIIa required to correct the bleeding following the saphenous vein injury in mouse hemophilia model systems. Higher doses of rFVIIa were required to restore hemostasis in EPCR-overexpressing hemophilia mice compared with hemophilia mice expressing normal levels of EPCR. Administration of FVIII antibody induced only mild hemophilic bleeding in EPCR-deficient mice, which was corrected completely with a low dose of rFVIIa. Administration of therapeutic concentrations of rFVIIa increased plasma protein C levels in EPCR-overexpressing mice, indicating the displacement of protein C from EPCR by rFVIIa. EPCR levels did not significantly alter the bioavailability of rFVIIa in plasma. Overall, our data indicate that EPCR levels influence the hemostatic effect of rFVIIa in treating hemophilia. Our present findings suggest that FVIIa displacement of anticoagulant protein C from EPCR that results in downregulation of activated protein C generation and not the direct effect of EPCR-FVIIa on factor X activation is the mechanism by which FVIIa interaction with EPCR contributes to the hemostatic effect of rFVIIa in hemophilia therapy.
Introduction
Recent studies from our laboratory1 and others2,3 have established that clotting factor VIIa (FVIIa), whose function is to initiate the coagulation cascade following its binding to tissue factor (TF),4 also binds endothelial cell protein C receptor (EPCR),5 a key protein in the activated protein C (APC)-mediated anticoagulant pathway.6 Protein C is the primary ligand for the EPCR, and EPCR binding promotes protein C activation by the thrombin:thrombomodulin complex.7 Human FVIIa and human protein C bind to human EPCR with similar affinities.1 Pharmacological concentrations of human rFVIIa were shown to downregulate the EPCR-mediated activation of protein C in the human endothelial cell model system.1 Murine FVIIa does not bind to either murine or human EPCR, but human FVIIa binds murine EPCR both in vitro and in vivo.8 Administration of a high concentration of human recombinant FVIIai (rFVIIai) (10 mg/kg) to EPCR-overexpressing mice, whose plasma protein C levels were lower because of much of protein C being associated with the vascular endothelium overexpressing EPCR, increased protein C levels in plasma markedly.8 These data suggest that exogenously administered FVIIa could displace protein C bound to EPCR in vivo. Because only a small fraction of protein C in the plasma is expected to be associated with EPCR in normal physiology, FVIIai administration resulted in only a small, not statistically significant, increase in protein C levels in plasma of wild-type mice.8
rFVIIa has been used widely for more than 2 decades to treat bleeding disorders in hemophilia patients with inhibitors and other groups of patients.9,10 Although a number of mechanisms have been proposed to explain the therapeutic effect of rFVIIa, either involving TF-dependent or platelet-dependent/TF-independent mechanisms,9,11-13 the mode of rFVIIa action in treating hemophilia is not entirely clear. We postulated earlier that FVIIa binding to EPCR might augment the hemostatic effect of rFVIIa in therapeutic conditions by effectively competing with protein C for limited EPCR on the endothelium and thus downregulating APC generation.1,5 However, recent studies from others suggest that FVIIa interaction with EPCR may also influence the hemostatic effect of rFVIIa through direct EPCR-FVIIa activation of factor X (FX) or EPCR tethering of FVIIa, providing an extended locale of procoagulant reactions on the endothelium.14 The present study is carried out to investigate potential mechanisms by which FVIIa interaction with EPCR contributes to the hemostatic effect of rFVIIa in hemophilia therapy using wild-type, EPCR-deficient (EPCR-def), and EPCR-overexpressing mice and inducing hemophilic condition in the mice by administration of FVIII antibody.
Materials and methods
Reagents
Human rFVIIa and active site-inhibited human rFVIIa (FVIIaAI) were provided by the late Walter Kisiel, University of New Mexico, Albuquerque, NM. FVIIaAI was prepared by blocking the active site of human rFVIIa with twofold molar excess of D-Phe-L-Phe-L-Arg chloromethyl ketone as described earlier.15 FVIIaAI has no detectable proteolytic activity. Human FVIII monoclonal antibody (mAb) that crossreacts with murine FVIII and inhibits murine FVIII activity (GMA 8015) was obtained from Green Mountain Antibodies (Burlington, VT). Preparation of murine APC and protein C antibody was described earlier.16
Mice
Wild-type mice (C57BL/6J) and FVIII−/− mice (B6/129S) were obtained from Jackson Laboratories (Bar Harbor, ME) or bred in-house. Generation of EPCR-def mice (Procr−/−) and EPCR-overexpressing mice (Tie2-EPCR) was described earlier.16,17 Both EPCR-def and Tie2-EPCR mice were bred in the C57BL/6J genetic background. Tie2-EPCR mice express at least eightfold higher EPCR protein in all tissues compared with wild-type mice.16 Soluble EPCR levels in plasma of Tie2-EPCR mice were 12-fold higher than of wild-type mice. No soluble EPCR was detected in EPCR-def mice. FVIII−/− mice in the B6/129S background were backcrossed with C57BL/6J mice for more than 10 generations to generate FVIII−/− mice in the C57BL/6J genetic background. Tie2-EPCR/FVIII−/− mice were generated by crossing Tie2-EPCR mice with FVIII−/− mice in the C57BL/6J genetic background. Except in experiments described in Figure 1, in which FVIII−/− mice in the B6/129S genetic background were used, all other experiments were carried out with mice in the C57BL/6J genetic background. All studies involving animals were conducted in accordance with the animal welfare guidelines set forth in the Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at Tyler.
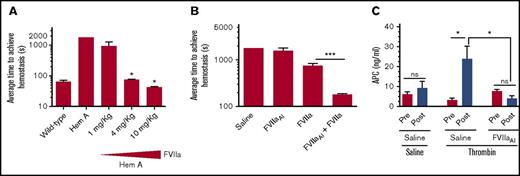
Proteolytically inactive FVIIa binding to EPCR augments the hemostatic activity of a low dose of rFVIIa in hemophilia A (Hem A) mice. (A) Average hemostatic times of wild-type and hemophilia A mice following saphenous vein incision and the effect of varying doses of rFVIIa in restoring hemostasis in hemophilia A mice. Saline or varying doses of rFVIIa (1, 4, or 10 mg/kg body weight) was administered to hemophilia A mice via the tail vein. Five minutes after rFVIIa administration, mice were subjected to saphenous vein incision. Average time to achieve hemostasis was determined as described in “Materials and methods.” *P < .05 compared with hemophilia mice not receiving rFVIIa. (B) Administration of a pharmacological concentration of FVIIaAI promotes the hemostatic effect of a low dose of rFVIIa. Hemophilia A mice were injected with saline, a low dose of rFVIIa (1 mg/kg), FVIIaAI (10 mg/kg), or both FVIIaAI (10 mg/kg) and rFVIIa (1 mg/kg). Five minutes following rFVIIa administration, mice were subjected to saphenous vein incision and the average time to achieve hemostasis was determined (n = 4-7 mice/group). ***P < .001. (C) FVIIaAI downregulation of APC generation. Wild-type mice were administered with saline or FVIIaAI (10 mg/kg) via the tail vein. After obtaining the blood sample (pre), mice were injected with saline or thrombin (6 U/kg) via the tail vein to induce activation of protein C in vivo. After 10 minutes, the blood was obtained (post). APC levels in plasma were measured as described earlier.16 Note: the data shown in panel A are essentially similar to that reported in our earlier publication19 and was reproduced here to illustrate the rationale behind using 1 mg/kg rFVIIa dose in experiments described in panel B. Hemophilia A mice used in this experiment were in the B6/129S genetic background. All other experiments were performed with mice in the C57BL/6J genetic background. NS, not significant.
Proteolytically inactive FVIIa binding to EPCR augments the hemostatic activity of a low dose of rFVIIa in hemophilia A (Hem A) mice. (A) Average hemostatic times of wild-type and hemophilia A mice following saphenous vein incision and the effect of varying doses of rFVIIa in restoring hemostasis in hemophilia A mice. Saline or varying doses of rFVIIa (1, 4, or 10 mg/kg body weight) was administered to hemophilia A mice via the tail vein. Five minutes after rFVIIa administration, mice were subjected to saphenous vein incision. Average time to achieve hemostasis was determined as described in “Materials and methods.” *P < .05 compared with hemophilia mice not receiving rFVIIa. (B) Administration of a pharmacological concentration of FVIIaAI promotes the hemostatic effect of a low dose of rFVIIa. Hemophilia A mice were injected with saline, a low dose of rFVIIa (1 mg/kg), FVIIaAI (10 mg/kg), or both FVIIaAI (10 mg/kg) and rFVIIa (1 mg/kg). Five minutes following rFVIIa administration, mice were subjected to saphenous vein incision and the average time to achieve hemostasis was determined (n = 4-7 mice/group). ***P < .001. (C) FVIIaAI downregulation of APC generation. Wild-type mice were administered with saline or FVIIaAI (10 mg/kg) via the tail vein. After obtaining the blood sample (pre), mice were injected with saline or thrombin (6 U/kg) via the tail vein to induce activation of protein C in vivo. After 10 minutes, the blood was obtained (post). APC levels in plasma were measured as described earlier.16 Note: the data shown in panel A are essentially similar to that reported in our earlier publication19 and was reproduced here to illustrate the rationale behind using 1 mg/kg rFVIIa dose in experiments described in panel B. Hemophilia A mice used in this experiment were in the B6/129S genetic background. All other experiments were performed with mice in the C57BL/6J genetic background. NS, not significant.
Hematological analysis of murine blood
For blood cell count analysis, blood was collected via submandibular vein in EDTA (2.7 mM) and analyzed using HEMAVET-HV950FS (Drew Scientific, Inc., CT) analyzer. Blood was collected in citrate anticoagulant for measuring plasma activated partial thromboplastin time (aPTT), dilute prothrombin time (PT), and FVIII clotting activities.
Saphenous vein bleeding
The saphenous vein bleeding model was described in detail in our recent report.13 Briefly, bleeding was induced by a sharp incision to the saphenous vein, and the bleeding from the cut was monitored for 30 minutes. After each hemostasis incident, the clot was disrupted gently to reinitiate a new bleeding episode. Average time to achieve hemostasis (ATH) was calculated from the number of clots formed during the 30-minute experimental period. Throughout the experimental duration, blood was adsorbed onto a filter paper, and blood volume on the filter was determined by measuring hemoglobin levels.
Assays
Standard clotting assay procedures were used to measure FVIII clotting activity, aPTT (Pacific Hemostasis Kontact APTT reagent, ThermoFisher Scientific), and dilute PT (1000× fold diluted Dade Innovin). APC and protein C levels in mouse plasma were determined as described earlier.16 rFVIIa antigen levels in plasma were determined as described earlier.18
Statistics
Statistical significance of differences between 2 groups was calculated using the nonparametric Mann-Whitney U test. ATH and blood loss in mice of different genotypes treated with the same concentration of rFVIIa were compared pairwise to evaluate the influence of EPCR on the rFVIIa-induced hemostatic effect.
Results
FVIIa binding to EPCR contributes to the hemostatic effect of rFVIIa in hemophilia independent of its proteolytic activity
In a recent study,19 we showed that treatment of hemophilia mice (FVIII−/− mice) with EPCR mAb that blocks protein C binding to EPCR reduced the amount of rFVIIa required to correct the bleeding. These data provided a proof-of-concept to the hypothesis that the downregulation of EPCR-mediated anticoagulation could augment the hemostatic effect of rFVIIa in hemophilia therapy. However, these data do not directly address whether the hemostatic effect achieved with high concentrations of rFVIIa in the treatment of hemophilia comes partly from FVIIa binding to EPCR or solely through direct activation of FX by rFVIIa. To investigate this, we administered a dose of rFVIIa that had no or only a minimal effect toward restoring hemostasis in hemophilia A mice, along with a high concentration of active site, FVIIaAI (10 mg/kg), which lacks the coagulant activity but is capable of binding EPCR.1 As shown in Figure 1A, administration of 1 mg/kg of rFVIIa to hemophilia A mice had a minimal effect on restoring hemostasis. Similarly, administration of 10 mg/kg of FVIIaAI alone had no significant effect on the bleeding time of hemophilia A mice (Figure 1B). However, a combination of a low dose of rFVIIa (1 mg/kg) and a high dose of FVIIaAI (10 mg/kg) effectively restored hemostasis in hemophilia A mice (Figure 1B). Our earlier studies showed that administration of the same concentration of rFVIIaAI increased protein C levels in plasma, indicating that FVIIa competes with endogenous protein C binding to EPCR and displaces protein C from the EPCR.8 Measurement of APC generation in these groups of mice following a defined amount of thrombin infusion to activate protein C showed reduced levels of APC in mice administered with FVIIaAI (Figure 1C).
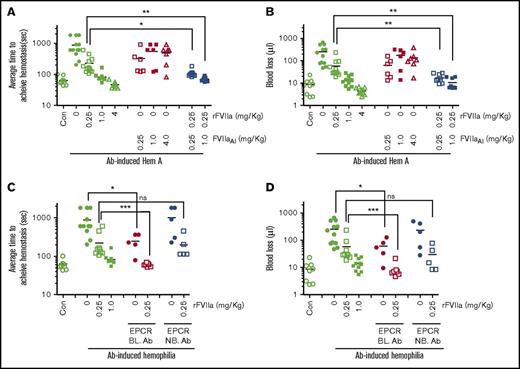
To further strengthen the relevance of FVIIa-EPCR interaction in the rFVIIa treatment of hemophilia, we employed FVIII antibody (Ab)-induced hemophilia mouse model and a dose of rFVIIa that was within the clinical dose range (0.25 mg/kg). As shown in Figure 2A, administration of FVIII mAb markedly prolonged the bleeding time in wild-type C57BL/6J mice (ATH in control mice was 63 ± 7 seconds and FVIII Ab administered mice was 876 ± 172 seconds). The administration of 1 and 4 mg/kg rFVIIa fully corrected the bleeding, whereas a dose of 0.25 mg/kg rFVIIa showed a partial reduction of bleeding in FVIII Ab-induced hemophilia mice. The difference in rFVIIa doses required to correct the bleeding in FVIII−/− mice (Figure 1A) vs Ab-induced hemophilia mice (Figure 2A) reflects differences in the severity of bleeding between these 2 models; FVIII antibodies did not completely neutralize FVIII activity and resulted in approximately 5% to 10% of residual FVIII activity. Administration of FVIIaAI alone, in doses of 0.25, 1, and 4 mg/kg, reduced the bleeding time partially in FVIII Ab-induced hemophilia mice compared with Ab-induced hemophilia mice receiving saline, but the reduction did not reach statistical significance. However, administration of 0.25 mg/kg rFVIIa along with FVIIaAI (0.25 or 1.0 mg/kg) significantly reduced the ATH in Ab-induced hemophilia mice (rFVIIa alone, 227 ± 44 seconds; rFVIIa + 0.25 mg/kg FVIIaAI, 106 ± 9.7 seconds; rFVIIa + 1.0 mg/kg FVIIaAI, 70 ± 4 seconds). Similar results were also noted when bleeding was evaluated as the volume of blood lost (Figure 2B). Blocking the EPCR with an EPCR mAb that blocks protein C binding to the EPCR in itself significantly reduced the bleeding in this model (ATH, 245 ± 53 seconds). Administration of 0.25 mg/kg rFVIIa fully restored the hemostasis in mice that received EPCR-blocking mAb (ATH, 59 ± 2 seconds; Figure 2C-D). Overall, these data indicate that blocking protein C binding to EPCR could promote hemostasis in hemophilia. These data imply that the hemostatic effect of rFVIIa in hemophilia therapy comes not only from FVIIa activation of FX, but also from FVIIa binding competitively to EPCR, which leads to displacement of protein C from EPCR and the subsequent downregulation of APC generation.
A low concentration of rFVIIa, 0.25 mg/kg, restores hemostasis in FVIII antibody-induced hemophilia mice administered with proteolytically inactive FVIIa or EPCR blocking antibodies (BL. Ab). (A,B) Wild-type C57BL/6J mice were administered intravenously with saline (Con) or FVIII mAb (1 mg/kg) to induce hemophilia. Two hours after administering FVIII antibody, the mice were injected with saline (0), 0.25, 1.0, or 4.0 mg/kg rFVIIa or FVIIAI, or 0.25 mg/kg rFVIIa plus FVIIAI (0.25 or 1.0 mg/kg) via the tail vein. Five minutes following rFVIIa administration, the bleeding was initiated by the saphenous vein incision and average time to achieve hemostasis (A) and blood loss (B) were determined. (C-D) FVIII antibody-induced hemophilia mice were injected with EPCR blocking or non-blocking antibodies (4 mg/kg) ± 0.25 mg/kg rFVIIa. Following the saphenous vein injury, the average time to achieve hemostasis (C) and blood loss (D) were determined as described in “Materials and methods” (n = 5-10 mice/group). *P < .05; **P < .01 ***P < .001. Note: The data shown for Con and Ab-induced hemophilia with 0, 0.25, and 1.0 mg/kg of rFVIIa (C-D) were the same as that of shown in panels A and B, respectively, except for minor differences in the number of animals in some groups.
A low concentration of rFVIIa, 0.25 mg/kg, restores hemostasis in FVIII antibody-induced hemophilia mice administered with proteolytically inactive FVIIa or EPCR blocking antibodies (BL. Ab). (A,B) Wild-type C57BL/6J mice were administered intravenously with saline (Con) or FVIII mAb (1 mg/kg) to induce hemophilia. Two hours after administering FVIII antibody, the mice were injected with saline (0), 0.25, 1.0, or 4.0 mg/kg rFVIIa or FVIIAI, or 0.25 mg/kg rFVIIa plus FVIIAI (0.25 or 1.0 mg/kg) via the tail vein. Five minutes following rFVIIa administration, the bleeding was initiated by the saphenous vein incision and average time to achieve hemostasis (A) and blood loss (B) were determined. (C-D) FVIII antibody-induced hemophilia mice were injected with EPCR blocking or non-blocking antibodies (4 mg/kg) ± 0.25 mg/kg rFVIIa. Following the saphenous vein injury, the average time to achieve hemostasis (C) and blood loss (D) were determined as described in “Materials and methods” (n = 5-10 mice/group). *P < .05; **P < .01 ***P < .001. Note: The data shown for Con and Ab-induced hemophilia with 0, 0.25, and 1.0 mg/kg of rFVIIa (C-D) were the same as that of shown in panels A and B, respectively, except for minor differences in the number of animals in some groups.
EPCR levels modulate bleeding propensity in acquired hemophilia and rFVIIa dose requirements to correct the bleeding disorder
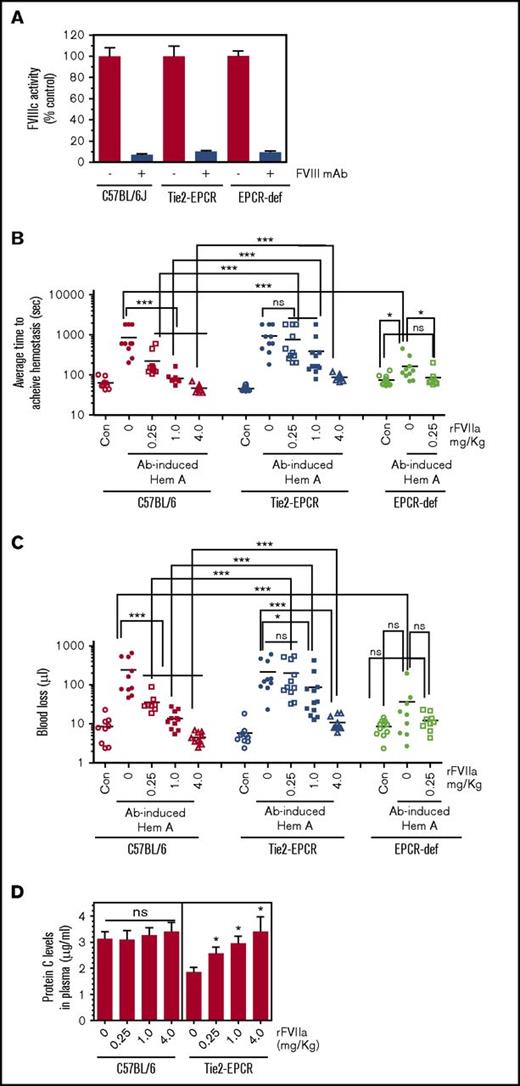
First, we investigated the role of EPCR in basal hemostasis by comparing bleeding episodes in wild-type, EPCR-def, and Tie2-EPCR (EPCR-overexpressing) mice following the saphenous vein incision. In this model, wild-type and EPCR-def mice exhibited similar bleeding times (wild-type, 63 ± 7 seconds; EPCR-def, 74 ± 6 seconds; n = 9 to 12 mice/group). The small difference between them is not statistically significant. Surprisingly, the mean ATH in Tie2-EPCR mice was significantly shorter (46 ± 2 seconds, n = 10) than that was noted in wild-type or EPCR-def mice. Analysis of blood components at baseline showed no significant differences among wild-type, EPCR-def, and Tie2-EPCR mice in their platelet, red blood cell, and other blood cell counts (data not shown). Measurement of aPTT and dilute PT also showed no significant differences among them (aPTT in seconds: wild-type, 48 ± 1.0; EPCR-def, 45 ± 1.2; Tie2-EPCR, 48 ± 0.9; dilute PT in seconds: wild-type, 29.5 ± 1.1; EPCR-def, 26.0 ± 0.7; Tie2-EPCR, 28.4 ± 0.7). These data were consistent with an earlier report demonstrating no significant differences in platelet count, thrombin–antithrombin complex, and fibrinogen levels between wild-type and Tie2-EPCR mice.16 In the absence of any apparent differences among the genotypes in their general coagulation profile and blood cell count, the reason for a shorter ATH observed in Tie2-EPCR mice is unknown at present.
Next, we induced acquired hemophilia in wild-type, EPCR-def, and Tie2-EPCR mice by injecting FVIII mAb (1 mg/kg). The antibody neutralized 90% to 95% of FVIII clotting activity in all 3 groups (Figure 3A). Administration of FVIII mAb markedly prolonged the bleeding time in wild-type mice as expected (from a median bleeding time of 55 seconds in control mice to 600 seconds in FVIII mAb–administered mice) and also in Tie2-EPCR mice (median bleeding time, 750 seconds) (Figure 3B). Interestingly, EPCR-deficient mice were protected to a large extent, but not completely, from the antibody-induced acquired hemophilia (median bleeding time: control EPCR-deficient mice, 67 seconds; FVIII mAb administered EPCR-deficient mice, 120 seconds; FVIII mAb administered wild-type mice, 600 seconds). Administration of a low dose of rFVIIa (0.25 mg/kg) fully corrected the mild bleeding defect of acquired hemophilia in EPCR-deficient mice (median bleed time, 67 seconds). As expected from Figure 2A, the administration of 0.25 mg/kg rFVIIa only partially corrected the bleeding disorder in wild-type mice with acquired hemophilia; a dose of 1 mg/kg of rFVIIa fully restored the hemostasis in these mice (Figure 3B). In Tie2-EPCR acquired hemophilia mice, administration of 0.25 mg/kg rFVIIa had no significant effect on the prolonged bleeding time. Even 4 mg/kg of rFVIIa failed to correct the bleeding phenotype in this group fully. At all doses of rFVIIa tested, the therapeutic effect of rFVIIa in restoring hemostasis in Tie2-EPCR mice was significantly lower than that was observed in wild-type acquired hemophilia mice. Similar results were observed when bleeding was evaluated as the volume of blood lost (Figure 3C). Measurement of protein C levels showed that administration of rFVIIa, at all doses, significantly increased plasma protein C levels in Tie2-EPCR mice (Figure 3D). As expected from an earlier study,8 we did not detect a significant increase in plasma protein C levels in Ab-induced hemophilia mice expressing normal levels of EPCR following rFVIIa administration (Figure 3D). Because only a minute fraction of protein C was expected to be associated with EPCR in wild-type mice, even a complete displacement of protein C from the EPCR would not significantly increase plasma protein C levels. To investigate whether the reduced hemostatic effect of rFVIIa in the Tie2-EPCR mice could be due to a potential reduction of rFVIIa in the circulation because of binding to overexpressed EPCR on the endothelium, we measured rFVIIa antigen levels in plasma of wild-type and Tie2-EPCR mice at 5 and 30 minutes following rFVIIa administration (0.25 mg/kg). We found no significant differences in rFVIIa levels in plasma between the 2 groups (rFVIIa, ng/mL, at 5 minutes: wild-type mice, 1105 ± 115; Tie2-EPCR mice, 887 ± 99; at 30 minutes: wild-type mice, 381 ± 46; Tie2-EPCR mice, 398 ± 24; n = 4).
EPCR levels modulate the hemostatic effect of rFVIIa in hemophilia. Wild-type, Tie2-EPCR, and EPCR-deficient mice were administered intravenously with saline or FVIII mAb (1 mg/kg) to induce hemophilia. Two hours after administering the antibody, the hemophilia-induced mice were injected with saline (0), 0.25, 1.0, or 4.0 mg/kg rFVIIa via the tail vein. Five minutes following rFVIIa administration, the bleeding was initiated by the saphenous vein incision and average time to achieve hemostasis and blood loss were determined. At the end of the experimental period, blood from a group of mice receiving saline or FVIII mAb but not treated with rFVIIa was obtained by cardiac puncture to isolate plasma to measure FVIII clotting activity. (A) FVIII clotting activity levels; (B) average time to achieve hemostasis; (C) blood loss (n = 9-12 mice/group). (D) Protein C levels in plasma of wild-type mice and Tie2-EPCR mice administered with varying concentrations of rFVIIa. Plasmas were obtained from mice groups (B) following the completion of bleeding analysis. *P < .05; **P < .01; ***P < .001. When performing the statistical comparison between rFVIIa effectiveness in restoring hemostasis in Ab-induced hemophilic wild-type and Tie2-EPCR mice, each dose of rFVIIa was individually compared between wild-type and Tie2-EPCR mice. Note: The data shown for Con and Ab-induced hemophilia with 0, 0.25, and 1.0 mg/kg of rFVIIa in wild-type mice (B-C) were essentially the same as shown in Figure 2A-B, respectively, except for minor differences in the number of animals in some groups.
EPCR levels modulate the hemostatic effect of rFVIIa in hemophilia. Wild-type, Tie2-EPCR, and EPCR-deficient mice were administered intravenously with saline or FVIII mAb (1 mg/kg) to induce hemophilia. Two hours after administering the antibody, the hemophilia-induced mice were injected with saline (0), 0.25, 1.0, or 4.0 mg/kg rFVIIa via the tail vein. Five minutes following rFVIIa administration, the bleeding was initiated by the saphenous vein incision and average time to achieve hemostasis and blood loss were determined. At the end of the experimental period, blood from a group of mice receiving saline or FVIII mAb but not treated with rFVIIa was obtained by cardiac puncture to isolate plasma to measure FVIII clotting activity. (A) FVIII clotting activity levels; (B) average time to achieve hemostasis; (C) blood loss (n = 9-12 mice/group). (D) Protein C levels in plasma of wild-type mice and Tie2-EPCR mice administered with varying concentrations of rFVIIa. Plasmas were obtained from mice groups (B) following the completion of bleeding analysis. *P < .05; **P < .01; ***P < .001. When performing the statistical comparison between rFVIIa effectiveness in restoring hemostasis in Ab-induced hemophilic wild-type and Tie2-EPCR mice, each dose of rFVIIa was individually compared between wild-type and Tie2-EPCR mice. Note: The data shown for Con and Ab-induced hemophilia with 0, 0.25, and 1.0 mg/kg of rFVIIa in wild-type mice (B-C) were essentially the same as shown in Figure 2A-B, respectively, except for minor differences in the number of animals in some groups.
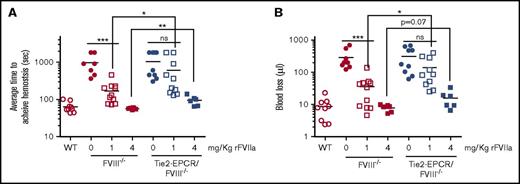
The reduced hemostatic effect of rFVIIa in EPCR-overexpressing mice is also evident in FVIII−/− genetic background mice (Figure 4A-B). Comparison of rFVIIa hemostatic effect in FVIII−/− and Tie2-EPCR/FVIII−/− mice showed that 1 mg/kg rFVIIa provided a partial, but consistent and statistically significant, protection against bleeding in FVIII−/− mice. However, this dose of rFVIIa failed to provide a significant reduction in the bleeding of Tie2-EPCR/FVIII−/− mice (Figure 4A). A dose of 4 mg/kg rFVIIa reduced the bleeding in both FVIII−/− and Tie2-EPCR/FVIII−/− mice, but the difference between them is statistically significant (Figure 4A). Unavailability of EPCR-def/FVIII−/− mice at present did not allow us to perform the similar experiments in EPCR-def/FVIII−/− mice.
Comparison of the hemostatic effect of rFVIIa in FVIII−/−and Tie2-EPCR/FVIII−/−mice. FVIII−/− mice or Tie2-EPCR/FVIII−/− mice were administered with 0, 1, or 4 mg/kg rFVIIa via the tail vein and subjected to the saphenous vein incision. The average time to achieve hemostasis (A) and blood loss (B) were measured as described in methods (n = 6 to 10 mice/group). * P < .05; ** P < .01; *** P < .001; ns, not statistically significant difference.
Comparison of the hemostatic effect of rFVIIa in FVIII−/−and Tie2-EPCR/FVIII−/−mice. FVIII−/− mice or Tie2-EPCR/FVIII−/− mice were administered with 0, 1, or 4 mg/kg rFVIIa via the tail vein and subjected to the saphenous vein incision. The average time to achieve hemostasis (A) and blood loss (B) were measured as described in methods (n = 6 to 10 mice/group). * P < .05; ** P < .01; *** P < .001; ns, not statistically significant difference.
Discussion
Although earlier studies suggest that FVIIa-EPCR interaction may contribute to the hemostatic effect of FVIIa in hemophilia therapy,5,14,20 it is not entirely clear the mechanism by which EPCR-FVIIa interaction contributes to the hemostatic effect. EPCR-FVIIa interaction could contribute to the hemostatic effect of FVIIa by competing with protein C for the EPCR and thus downregulating EPCR-mediated APC generation,1,5,19 by FVIIa-EPCR directly activating FX, or by EPCR tethering of FVIIa on the endothelium thus extending the locale of procoagulant reactions.14 The present study that employed inactive FVIIa and mice lacking or overexpressing EPCR supports the concept that EPCR-FVIIa interaction contributes to the hemostatic effect in hemophilia by modulating EPCR-dependent anticoagulant pathway. Our earlier studies that showed a marked increase in rFVIIa binding to vascular endothelium in Tie2-EPCR mice compared with wild-type mice and no detectable binding in EPCR-deficient mice demonstrate that human rFVIIa administered to mice readily associates with mouse EPCR on the endothelium.8 If EPCR influences the hemostatic effect of rFVIIa because FVIIa-EPCR complexes could directly activate FX or because EPCR tethering of rFVIIa on the endothelium extends the locale of procoagulant reactions, then one would expect an increased hemostatic effect of rFVIIa in Tie2-EPCR hemophilia mice. Contrary to this, we found here that the increased EPCR expression dampens the hemostatic effect of rFVIIa. Generation of increased levels of APC following initial thrombin generation may be responsible for the reduced hemostatic effect of rFVIIa in Tie2-EPCR mice.
Our present data that show EPCR-deficient mice are protected to a large extent from acquired hemophilia suggests that sufficient prothrombinase activity could be formed even in hemophilia to generate necessary thrombin to sustain hemostasis, but the EPCR-dependent APC generation effectively blocks this process by inactivating the prothrombinase. These data suggest that EPCR-mediated APC anticoagulant pathway plays a critical role in modulating thrombin generation in hemophilia and inhibition of APC anticoagulant pathway can balance the deficiency in the procoagulant pathways in hemophilia. Recent observations that showed inhibition of APC with a variant of α1-antitrypsin21 or specific antibody restored hemostasis in mouse and monkey bleeding model systems, respectively, are consistent with the above concept. The ability of super FVa (FVa resistant to APC inactivation) to correct the blood loss in hemophilia mice22,23 also supports the above concept.
On the face value, the use of supra-clinical doses of rFVIIa and FVIIaAI used in the mouse hemophilia model system raises a caveat in extrapolating the present data to define the mode of rFVIIa action in human hemophilia treatment. It is unlikely that the differences between human and murine FVIIa to interact with murine TF or EPCR alone were the reasons for this discrepancy since murine FVIIa variants that effectively interact with both TF and EPCR were also used at 3 mg/Kg dose to restore hemostasis in hemophilia B mice.14,20 The restoration of hemostasis in FVIII Ab-induced hemophilia, where the bleeding phenotype is not as severe as in FVIII genetic deficient mice (FVIII−/− mice) because of residual FVIII activity in the Ab-induced hemophilia model, required 4 to 10 times less rFVIIa (0.25 to 1 mg/kg) than necessary in FVIII−/− mice (1 to 10 mg/kg).
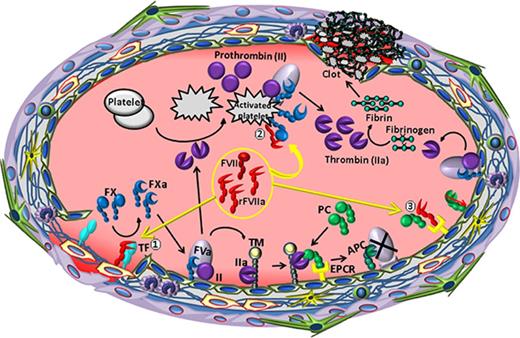
The apparent dosage differences in restoring hemostasis in mouse and human hemophilia may reflect differences in pharmacokinetics and pharmacodynamics between the 2 species. Scaling of the dose based on the body weight alone is perceived incorrect since the differences in pharmacokinetics and pharmacodynamics among species greatly influence dose estimations.24,25 The allometric approach that accounts for the differences in body surface area, metabolic rates, and physiological processes among species is recommended for dose conversion between species.25,26 Based on this approach, 90 to 270 µg/kg dose, the clinically relevant dose range of rFVIIa in treating hemophilia patients would be equivalent to that of 1.1 to 3.32 mg/kg dose in the mouse. Thus, the rFVIIa dose used to restore hemostasis in mouse hemophilia model in the present study is within clinically relevant human equivalent dose range. Therefore, our present data support the hypothesis that in therapeutic conditions binding to rFVIIa to EPCR promotes the hemostatic effect of rFVIIa through the downregulation of EPCR-mediated anticoagulation by displacing protein C from the EPCR, and not because of EPCR-FVIIa directly activating FX (Figure 5).
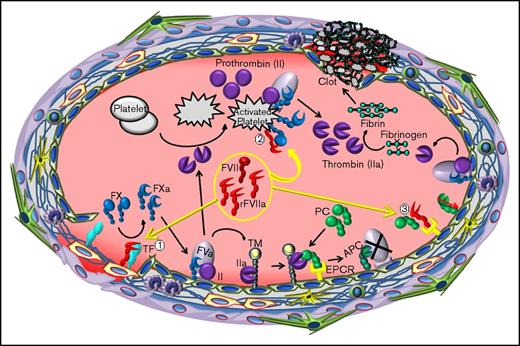
The relevance of FVIIa and EPCR interaction to the hemostatic effect of rFVIIa in hemophilia therapy. When high concentrations of rFVIIa were administered to hemophilia patients with inhibitors, rFVIIa, along with plasma zymogen FVII, binds to tissue factor (TF) at the injury site (1). The formation of the TF-FVIIa complex initiates the activation of FX to FXa and also FIX to FIXa. Once traces of FXa are generated, tissue factor pathway inhibitor (TFPI) binds FXa and TFPI-FXa forms a quaternary complex with TF-FVIIa, inhibiting further activation of FX by TF-FVIIa (not shown). The initial FXa generated by TF-FVIIa also associates with factor Va (FVa) on the endothelial cell surface to form the prothrombinase complex, which activates prothrombin (FII) to thrombin. The resultant thrombin promotes the activation of protein C bound to the EPCR on the endothelium and also activates platelets. EPCR-mediated APC generation inactivates FVa and blocks further thrombin generation by the prothrombinase complex on the endothelium. However, rFVIIa, when present at high concentrations, can activate FX to FXa on the surface of thrombin-activated platelets, independent of TF (2). FXa generated by FVIIa on activated platelets forms appreciable levels of the prothrombinase complex that could lead to thrombin burst capable of restoring hemostasis in hemophilia patients. rFVIIa, in addition to activating FX, also displaces protein C from EPCR by directly competing with protein C to bind to the EPCR (3). FVIIa displacement of protein C from the EPCR reduces APC generation. Reduced APC levels would diminish the extent of FVa inactivation by APC, which would lead to sustained thrombin generation on the endothelium. This, in addition to rFVIIa directly activating FX, contributes to rFVIIa hemostatic effect in hemophilia therapy.
The relevance of FVIIa and EPCR interaction to the hemostatic effect of rFVIIa in hemophilia therapy. When high concentrations of rFVIIa were administered to hemophilia patients with inhibitors, rFVIIa, along with plasma zymogen FVII, binds to tissue factor (TF) at the injury site (1). The formation of the TF-FVIIa complex initiates the activation of FX to FXa and also FIX to FIXa. Once traces of FXa are generated, tissue factor pathway inhibitor (TFPI) binds FXa and TFPI-FXa forms a quaternary complex with TF-FVIIa, inhibiting further activation of FX by TF-FVIIa (not shown). The initial FXa generated by TF-FVIIa also associates with factor Va (FVa) on the endothelial cell surface to form the prothrombinase complex, which activates prothrombin (FII) to thrombin. The resultant thrombin promotes the activation of protein C bound to the EPCR on the endothelium and also activates platelets. EPCR-mediated APC generation inactivates FVa and blocks further thrombin generation by the prothrombinase complex on the endothelium. However, rFVIIa, when present at high concentrations, can activate FX to FXa on the surface of thrombin-activated platelets, independent of TF (2). FXa generated by FVIIa on activated platelets forms appreciable levels of the prothrombinase complex that could lead to thrombin burst capable of restoring hemostasis in hemophilia patients. rFVIIa, in addition to activating FX, also displaces protein C from EPCR by directly competing with protein C to bind to the EPCR (3). FVIIa displacement of protein C from the EPCR reduces APC generation. Reduced APC levels would diminish the extent of FVa inactivation by APC, which would lead to sustained thrombin generation on the endothelium. This, in addition to rFVIIa directly activating FX, contributes to rFVIIa hemostatic effect in hemophilia therapy.
Both zymogen FVII and FVIIa bind EPCR with a similar affinity as of protein C and APC.1 Because of a much lower molar concentration of zymogen FVII (10 nM) in plasma compared with protein C (60 nM), endogenous zymogen FVII would have only a minor effect on replacing protein C from EPCR. Administration of clinical doses of rFVIIa (90 to 270 µg/kg) may result in at the most 1.8 to 5.4 µg/mL (36 to 108 nM) rFVIIa in plasma, and thus elevates the total concentration of FVII(a) to 46 to 118 nM. Under these conditions, rFVIIa could reduce protein C binding to EPCR by about 40% to 65%. Therefore, clinical doses of rFVIIa may significantly impair EPCR-mediated APC generation. If so, an rFVIIa variant that could bind EPCR with a higher affinity than wild-type rFVIIa may act as a preferred hemostatic agent in treating bleeding disorders.
In the present study, we have used FVIIaAI as a tool to differentiate the effect of FVIIa binding to EPCR in promoting the hemostatic effect vs the hemostatic effect conferred by FVIIa activation of FX. Our present observation that administration of FVIIaAI promotes the procoagulant effect in hemophilia mouse model should not be misconstrued as conflicting with earlier observations that showed FVIIaAI acts as an antithrombotic agent in thrombosis model systems.27-29 In thrombosis model systems, circulating blood is exposed to TF in blood vessel wall following injury/insult, and the resultant TF-FVIIa complexes activate FX that lead to excessive thrombin generation and intravascular thrombosis. FVIIaAI, which binds TF with very high affinity,15 could effectively block TF-FVIIa complex formation and thus inhibit TF-dependent thrombus formation. Studies from our laboratory13 and others30 showed that the hemostatic effect of rFVIIa in hemophilia stems from the TF-independent mechanism. FVIIa binding to phospholipids exposed on the activated platelets and the platelet-bound FVIIa activation of FX are thought to be responsible for the hemostatic effect of rFVIIa in hemophilia patients.31,32 Given the low affinity of FVIIa binding to phospholipids and activated platelets,33 it is unlikely that FVIIaAI affects FVIIa binding to activated platelets to block platelet/phospholipid-dependent FVIIa-induced procoagulant response. However, under a similar condition, FVIIaAI can displace protein C from EPCR on the vascular endothelium.
EPCR expression is found on vascular endothelium of large blood vessels,34 capillaries,35 and other cell types.5 However, EPCR expression in various vascular beds may not be uniform.34,35 Therefore, EPCR-dependent modulation of FVIIa hemostatic effect may only apply to bleeding locations where EPCR is expressed.
Finally, it is important to point out that similarly to APC, FVIIa is also capable of inducing protective cell signaling upon binding to EPCR through activation of protease-activated receptor-1.36,37 Therefore, suppression of EPCR-mediated APC generation by rFVIIa will not compromise EPCR-mediated cytoprotective effects. This property of FVIIa makes it a more desirable approach for use in the prophylactic treatment of hemophilia.
Acknowledgments
The authors thank Sion Williams for proofreading the manuscript.
This work was supported by grants from the National Institutes of Health, National Heart, Lung, and Blood Institute (HL107483 [L.V.M.R.] and UM1 HL120877 [C.T.E.]).
Authorship
Contribution: S.K. and J.S. performed experiments and analyzed data; A.R. bred mice and assisted in performing experiments; C.T.E. provided the reagents; L.V.M.R. conceived and designed the research, analyzed the data, and wrote the manuscript; U.R.P. contributed to the design of experiments; and all authors read and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: L. Vijaya Mohan Rao, Department of Cellular and Molecular Biology, The University of Texas Health Science Center at Tyler, 11937 US Hwy 271, Tyler, TX 75708-3154; e-mail: vijay.rao@uthct.edu.