Key Points
Rituximab is effective in preventing relapse in TTP patients in remission with low ADAMTS13 levels.
Reduced-dose rituximab (200 mg) is associated with higher rates of re-treatment than the standard dose (375 mg/m2).
Abstract
Acute antibody-mediated thrombotic thrombocytopenic purpura (TTP) is a thrombotic microangiopathy with high morbidity and mortality. Rituximab is highly effective as prophylaxis in patients at risk of acute TTP relapse, but the ideal dosing regimen is unknown. A multicenter retrospective cohort study evaluated outcomes of patients given rituximab prophylaxis to prevent TTP relapse. Rituximab was given in 76 episodes to 45 patients (34 women and 11 men). Four once-per-week infusions of standard- (375 mg/m2 [24 episodes]), reduced- (200 mg [19 episodes]), and intermediate- (500 mg [17 episodes]) dose rituximab were given; in the remaining 16 episodes, patients received 100 to 1000 mg rituximab in 1 to 5 doses. Patients were deemed at high risk of TTP relapse on the basis of ADAMTS13 activity dropping to ≤15% from the normal range. Preprophylaxis median ADAMTS13 level was 5% (range, <5% to 17%). Normalization of ADAMTS13 occurred in 78.9% of patients, with 92.1% having at least a partial response (ADAMTS13 ≥30%); 3 patients had no response. Over a median of 15 months (range, 1-141 months), there were only 3 TTP relapses (2 of these subacute) in the reduced dose group. Re-treatment with rituximab occurred in 50% of patient episodes at a median of 17.5 months (range, 9-112 months) after initial prophylaxis. There was a statistically higher rate of re-treatment in the reduced- vs standard-dose group: 0.38 vs 0.17 episodes per year, respectively. Treatment was generally well tolerated, infusional effects being the most commonly reported. Rituximab therapy is effective as prophylaxis for normalizing ADAMTS13 and is an additional measure for preventing acute TTP relapses in patients with immune TTP.
Introduction
Thrombotic thrombocytopenic purpura (TTP) is an aggressive, multisystem thrombotic microangiopathy that typically targets the central nervous system and heart. The majority of cases are mediated by immunoglobulin G (IgG) antibodies targeting the metalloprotease ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13).1,2 ADAMTS13 is critical to the cleavage of von Willebrand factor. Deficiency of ADAMTS13 leads to platelet aggregation and formation of microthrombi. Untreated, TTP has a mortality rate in excess of 90%. Although the combination of plasma exchange (PEX) and immunosuppression results in a reduction in mortality to 10% to 20%, this approach is associated with high early relapse rates of ∼20% to 50%.3-6
Rituximab (MabThera; Roche Pharmaceuticals), a humanized anti-CD20 monoclonal antibody, has been shown to be effective in a variety of other clinical settings, including in the treatment of autoimmune diseases7 and antibody-mediated hematologic disorders.8,9 In the setting of TTP, rituximab was initially used in relapsed or refractory patients and was found to be effective in attaining remission and reducing relapse rates.10,11 Subsequently, it was found to be efficacious in prolonging disease-free survival when given to patients in the acute setting compared with historical controls treated with PEX and steroids alone.3 Rituximab given as prophylaxis has also been found to be effective in rescuing patients at imminent risk of an acute TTP relapse, identified through detection of low ADAMTS13 levels, typically <10% to 15%.11-13 We have previously demonstrated that prophylactic administration of a standard dose of rituximab (375 mg/m2 once per week for 4 weeks) to patients with low ADAMTS13 levels (<15%) resulted in recovery of ADAMTS13 into the normal range in all but 1 of 17 patient episodes; there were no acute relapses in this group over a median follow-up period of 23 months.14 It has been confirmed that rituximab prophylaxis reduced relapse incidence from 0.57 episodes per year to 0 episodes per year when compared with historical controls.15
Despite the efficacy of rituximab as TTP prophylaxis, the ideal dosing regimen remains unclear. Experience in other hematologic disorders (such as idiopathic thrombocytopenic purpura) suggests that using a reduced dose (such as 100 mg × 4 infusions) may be as efficacious as standard dosing.16,17 Furthermore, although rituximab is generally well tolerated, there is the potential for toxicity, including infusional side effects and hepatitis B reactivation18 ; repeated dosing may also lead to hypogammaglobulinemia.19 Thus, there is a need to investigate the efficacy of reduced-dose regimens in TTP to determine whether outcomes are comparable to those with a standard dose, with the possibility of reducing exposure to high doses and associated toxicity.
We undertook a multicenter retrospective cohort study to investigate the role of rituximab prophylaxis in preventing TTP relapse and the effect of dosage on ADAMTS13 recovery and treatment-free survival.
Patients and methods
We performed a retrospective analysis of all TTP patients who received rituximab prophylaxis at 6 United Kingdom specialist TTP centers between 2005 and 2016, with the primary aim of assessing the efficacy of rituximab in preventing acute TTP relapse and comparing outcomes with different dosing regimens. For all patients, we investigated rates of ADAMTS13 recovery and treatment-free survival after prophylaxis. We also assessed treatment-related toxicity.
Patients
Patients were identified from the United Kingdom TTP Study Registry (a database and Biobank of United Kingdom TTP-Multicentre Research Ethics Committee [MREC]: 08/H0810/54). This group comprised unselected consecutive patients with TTP in remission, all of whom had had at least 1 previous acute TTP episode. They were treated with rituximab prophylaxis because they were at high risk of relapse on the basis of low ADAMTS13 levels detected via routine monitoring samples taken in the outpatient clinic. Patients consented to having their clinical and laboratory data reviewed (MREC: 08/H0810/54, MREC: 08/H0716/72). A local medical research ethics committee provided approval. TTP was diagnosed on the basis of national guidelines.20 Remission was defined as a sustained platelet count of >150 × 109/L for 2 consecutive days. Relapse was defined as readmission with thrombocytopenia (platelet count of <150 × 109/L) with or without new symptoms 30 days after discharge from an acute episode.
Criteria for rituximab prophylaxis and pre-rituximab screening
Patients were given rituximab prophylaxis on the basis of reduced ADAMTS13 activity levels, which were confirmed on a separate sample (taken within 2 weeks of first sample) in patients who had a previous acute TTP episode associated with normalization of ADAMTS13 activity on remission. The majority of patients were given rituximab if their ADAMTS13 activity was ≤15%; 2 patients with higher ADAMTS13 levels (16% and 17%) were included because they were deemed to be at high risk of relapse on the basis of previous individual patient episodes and relapse history. No other immunosuppressive therapy was started at the time of rituximab prophylaxis; 2 patients were already taking mycophenolate mofetil (MMF), which was continued at the same dose (no increase) at the time of receiving rituximab. No patients were given maintenance rituximab. All patients underwent serologic hepatitis B screening pre-rituximab to identify those at risk of hepatitis B reactivation. No at-risk patients were excluded from receiving rituximab, but they were given lamivudine prophylaxis (100 mg once per day orally) for 6 months after rituximab treatment if they were at risk of reactivation of hepatitis B. Viral loads were monitored throughout treatment, and there were no cases of reactivation.
Rituximab dosing
Rituximab dosing regimens were recorded for all patients in a total of 76 patient episodes, including dose and number of doses given. For 60 patient episodes, 1 of 3 dosing regimens was used, defined as standard dose (375 mg/m2 given once per week for 4 weeks), reduced dose (200 mg once per week for 4 weeks), and intermediate dose (500 mg once per week for 4 weeks). Rituximab doses were not selected as a result of patient-related factors, and no pretreatment laboratory data were used to make decisions regarding dosing. Standard-dose rituximab was typically given at the beginning of the study when patients were given the dose used to treat lymphoma (375 mg/m2). After this, reduced-dose regimens (200 mg) were used because of the finding of possible benefit of this approach in other autoimmune disorders (including ITP). More recently, intermediate doses (500 mg) have been used. In the remaining 16 episodes, patients received 100 to 1000 mg rituximab in 1 to 5 doses.
Follow-up and outcome measures
All patients given rituximab prophylaxis were actively followed up with regular ADAMTS13 monitoring (at least once per month) until ADAMTS13 recovery and thereafter per clinicians’ routine protocol. Outcome measures used to assess efficacy in these patients were normalization of ADAMTS13 activity levels, relapse rates, and whether patients required re-treatment with rituximab prophylaxis (based on a subsequent decrease in ADAMTS13 levels using the same criteria). A complete response (CR) was defined as ADAMTS13 recovering into the normal range (≥60%), and a partial response (PR) was defined as ADAMTS13 recovering to ≥30% to 59%. If patients were re-treated with rituximab, the time interval between initial prophylaxis and subsequent prophylaxis was recorded.
Treatment-related toxicity
Infusional adverse effects (AEs) and other toxicity related to treatment were recorded for each patient and during outpatient follow-up visits, including infections, hepatitis B reactivation, and hypogammaglobulinemia.
ADAMTS13 assays and CD19 measurement
ADAMTS13 activity was analyzed by modification of the fluorescence resonance energy transfer assay (normal range, 60% to 123%).21 Anti-ADAMTS13 immunoglobulin G (IgG) levels were analyzed by using an in-house enzyme-linked immunosorbent assay technique as previously described (threshold for positivity, >6.1%).11,22 CD19 levels were measured by using a Navios flow cytometer (normal range, 4% to 26%) (Beckman Coulter).
Statistical analysis
For normally distributed data, 2-sample Student t tests were used to assess differences among groups. The Mann-Whitney U test was used for nonparametric data, and Fisher’s exact test was used to compare rates of ADAMTS13 recovery and rates of re-treatment among different rituximab dose groups. Time to re-treatment with rituximab was compared by using Kaplan-Meier estimates for right-censored data, and the log-rank test was used to compare treatment-free survival among patients in different rituximab dose groups.
Results
Patient demographics
During the 11-year period from 2005 to 2016, rituximab prophylaxis was given in 76 patient episodes to 45 patients (34 female, 11 male). Of these 45 patients, 14 (31.1%) had 1 previous acute TTP episode; the remaining 31 (68.9%) had more than 1 acute episode (range, 2-6 episodes). Female patients were treated in 60 patient episodes and males in 16 episodes (ratio of 3.75:1), with a median age of 43.5 years (range, 18-78 years) (Table 1). Patients’ ethnicity was Afro-Caribbean (29 episodes), white (27 episodes), Southeast Asian (13 episodes), or other (5 episodes); ethnicity was not recorded for 2 episodes.
Demographic characteristics and laboratory parameters of patients receiving rituximab prophylaxis for all 76 patients in total and 4 dose subgroups
| Characteristic . | Total (N = 76) . | Standard dose (375 mg/m2 × 4) (n = 24) . | Reduced dose (200 mg × 4) (n = 19) . | Intermediate dose (500 mg × 4) (n = 17) . | Other doses (n = 16) . |
|---|---|---|---|---|---|
| Female:male | 60:16 | 17:7 | 15:4 | 13:4 | 14:2 |
| Median age (range), y | 43.5 (18-78) | 41 (24-67) | 41 (18-74) | 46 (29-78) | 47 (19-65) |
| Median Hb (range), g/L | 133 (81*-171) | 135 (96-171) | 139 (81*-152) | 140 (123-163) | 125 (101-149) |
| Median platelets (range), × 109/L | 268 (83†-443) | 228 (109‡-333) | 287 (150-397) | 286 (199-443) | 306 (83†-390) |
| Median ADAMTS13 activity (range), % | 5 (<5-17) | <5 (<5-16) | 6 (<5-14) | 6 (<5-15) | 7 (<5-17) |
| Characteristic . | Total (N = 76) . | Standard dose (375 mg/m2 × 4) (n = 24) . | Reduced dose (200 mg × 4) (n = 19) . | Intermediate dose (500 mg × 4) (n = 17) . | Other doses (n = 16) . |
|---|---|---|---|---|---|
| Female:male | 60:16 | 17:7 | 15:4 | 13:4 | 14:2 |
| Median age (range), y | 43.5 (18-78) | 41 (24-67) | 41 (18-74) | 46 (29-78) | 47 (19-65) |
| Median Hb (range), g/L | 133 (81*-171) | 135 (96-171) | 139 (81*-152) | 140 (123-163) | 125 (101-149) |
| Median platelets (range), × 109/L | 268 (83†-443) | 228 (109‡-333) | 287 (150-397) | 286 (199-443) | 306 (83†-390) |
| Median ADAMTS13 activity (range), % | 5 (<5-17) | <5 (<5-16) | 6 (<5-14) | 6 (<5-15) | 7 (<5-17) |
Patient had low hemoglobin (Hb) because of coexisting iron deficiency anemia with no features of acute TTP.
Patient had a subacute TTP relapse.
Patient with platelets 109 × 109/L as a result of platelet clumping (evident on a blood film) with no features of microangiopathic hemolytic anemia.
Rituximab dose
Of the 76 overall patient episodes, 60 patients (78.9%) were treated with either standard- (24 [31.6%] of 76), reduced- (19 [25%] of 76), or intermediate-dose (17 [22.4%] of 76) rituximab (Table 1). The remaining 16 (21.1%) of 76 patients were treated with other dose regimens outlined in Table 2.
Dosing regimens for 16 patients who were not treated with standard-, reduced-, or intermediate-dose rituximab
| Rituximab dose . | No. of doses . | No. of patients . |
|---|---|---|
| 375 mg/m2 | 5 | 1 |
| 375 mg/m2 | 3 | 1 |
| 375 mg/m2 | 2 | 2 |
| 500 mg | 2 | 4 |
| 500 mg | 1 | 1 |
| 200 mg | 5 | 1 |
| 100 mg/m2 | 4 | 1 |
| 100 mg | 4 | 2 |
| Rituximab dose . | No. of doses . | No. of patients . |
|---|---|---|
| 375 mg/m2 | 5 | 1 |
| 375 mg/m2 | 3 | 1 |
| 375 mg/m2 | 2 | 2 |
| 500 mg | 2 | 4 |
| 500 mg | 1 | 1 |
| 200 mg | 5 | 1 |
| 100 mg/m2 | 4 | 1 |
| 100 mg | 4 | 2 |
There were 3 other patients: 2 patients had 200 mg × 2, then 500 mg × 1; 1 patient had 375 mg/m2 × 4, then 500 mg × 2.
Laboratory parameters
Laboratory parameters for the 76 patient episodes overall (total), and for the 4 different dose subgroups are provided in Table 1. Median hemoglobin (Hb) at the time of prophylactic therapy was 133 g/L (range, 81-171 g/L). Hb was noted to be below the laboratory lower limit of normal (<115 g/L) in 8 patient episodes (7 patients). The patient having the lowest documented Hb (2 episodes; Hb 81 g/L and 96 g/L) was known to have ongoing iron deficiency anemia; the remaining 6 patients had Hb levels of >100 g/L. No patient had evidence of fragmentation or hemolysis. The median platelet count at the time of prophylactic therapy was 268 × 109/L (range 83-443 × 109/L). The platelet count was below the lower limit of normal (<150 × 109/L) in 4 patient episodes (3 patients). In 3 episodes in 2 patients (platelet counts, 83 × 109/L, 125 × 109/L, and 119 × 109/L), these represented subacute TTP relapses and were classified as subacute based on the presence of thrombocytopenia (platelet count, <150 × 109/L) in the absence of symptoms and did not require treatment with PEX. The remaining patient episode (platelet count, 109 × 109/L) occurred as a result of platelet clumping (evident on a blood film) with no features of microangiopathic hemolytic anemia.
ADAMTS13 and anti-ADAMTS13 IgG level
Median ADAMTS13 level at the time of prophylactic therapy was 5% (range, <5% to 17%). All patients had ADAMTS13 levels of ≤15% at time of receiving rituximab prophylaxis except for 2 patients who had ADAMTS13 levels of 16% and 17%. Median anti-ADAMTS13 level before prophylaxis was 9% (range, 1% to 76%); 18 patients had an anti-ADAMTS13 antibody level below the positive cutoff of <6.1% (range, 1% to 5%), but all patients had a history of anti-ADAMTS13 antibodies detected during previous acquired acute TTP episodes.
Recovery of ADAMTS13 levels after rituximab prophylaxis
In 60 (78.9%) of 76 patient episodes, ADAMTS13 levels fully recovered into the normal range (CR, ≥60%), with median ADAMTS13 of 81% (range, 60% to 118%) at a median of 1 month after the first rituximab infusion (range, <1 to 5 months) (Table 3). Of the remaining 16 patient episodes, 10 were associated with ADAMTS13 recovery to at least 30% normal (PR) with a median ADAMTS13 of 47.5% (range, 30% to 58%) at a median of <1 month (range, <1 to 3 months). In total, 70 (92.1%) of 76 had an increase in ADAMTS13 to at least 30% (range, 30% to 118%). Of the remaining 6 patient episodes, 2 patients had an increase in ADAMTS13 activity to 23%, 3 had no ADAMTS 13 activity response, and 1 was lost to follow-up. Of the 3 patients who did not have a significant ADAMTS13 response, 1 was already receiving MMF at the time of rituximab prophylaxis. He was monitored and was subsequently found to have an increase in ADAMTS13 activity to 24% at 9 months after treatment. The other 2 nonresponders were started on other immunosuppressive agents: one was given MMF, and the other was given bortezomib. None of the 3 nonresponders had an acute relapse.
Outcome measures for patients receiving rituximab prophylaxis
| Measure . | Total (N = 76) . | Standard dose (n = 24) . | Reduced dose (n = 19) . | Intermediate dose (n = 17) . | Other doses (n = 16) . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n/N . | % . | Months (range) . | n/N . | % . | Months (range) . | n/N . | % . | Months (range) . | n/N . | % . | Months (range) . | n/N . | % . | Months (range) . | |
| No. of patients in CR (ADAMTS13 ≥60%) | 60/76 | 78.9 | 18/24 | 75 | 16/19 | 84.2 | 12/17 | 70.6 | 14/16 | 87.5 | |||||
| No. of patients in PR (ADAMTS13 30%-59%) | 10/76 | 13.2 | 3/24 | 12.5 | 2/19 | 10.5 | 4/17 | 23.5 | 1/16 | 6.25 | |||||
| No. of patients achieving at least a PR (ADAMTS13 ≥30%) | 70/76 | 92.1 | 21/24 | 87.5 | 18/19 | 94.7 | 16/17 | 94.1 | 15/16 | 93.4 | |||||
| Time to ADAMTS13 recovery | 1 (<1-5) | 1 (<1-5) | 2 (<1-4) | 1 (<1-3) | 2 (<1-4) | ||||||||||
| Median overall follow-up | 15 (1-141) | 17.5 (1-141) | 25 (9-43) | 10 (3-20) | 21 (3-112) | ||||||||||
| No. of patients requiring re-treatment | 38/76 | 50 | 12/24 * | 50 | 14/19 * | 73.7 | 3/17 | 17.6 | 10/16 | 62.5 | |||||
| Re-treatment episodes per year | 0.25 | 0.17† | 0.38† | 0.20 | 0.29 | ||||||||||
| Measure . | Total (N = 76) . | Standard dose (n = 24) . | Reduced dose (n = 19) . | Intermediate dose (n = 17) . | Other doses (n = 16) . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n/N . | % . | Months (range) . | n/N . | % . | Months (range) . | n/N . | % . | Months (range) . | n/N . | % . | Months (range) . | n/N . | % . | Months (range) . | |
| No. of patients in CR (ADAMTS13 ≥60%) | 60/76 | 78.9 | 18/24 | 75 | 16/19 | 84.2 | 12/17 | 70.6 | 14/16 | 87.5 | |||||
| No. of patients in PR (ADAMTS13 30%-59%) | 10/76 | 13.2 | 3/24 | 12.5 | 2/19 | 10.5 | 4/17 | 23.5 | 1/16 | 6.25 | |||||
| No. of patients achieving at least a PR (ADAMTS13 ≥30%) | 70/76 | 92.1 | 21/24 | 87.5 | 18/19 | 94.7 | 16/17 | 94.1 | 15/16 | 93.4 | |||||
| Time to ADAMTS13 recovery | 1 (<1-5) | 1 (<1-5) | 2 (<1-4) | 1 (<1-3) | 2 (<1-4) | ||||||||||
| Median overall follow-up | 15 (1-141) | 17.5 (1-141) | 25 (9-43) | 10 (3-20) | 21 (3-112) | ||||||||||
| No. of patients requiring re-treatment | 38/76 | 50 | 12/24 * | 50 | 14/19 * | 73.7 | 3/17 | 17.6 | 10/16 | 62.5 | |||||
| Re-treatment episodes per year | 0.25 | 0.17† | 0.38† | 0.20 | 0.29 | ||||||||||
n/N, number/total.
There was no significant difference in proportion of patients requiring re-treatment in standard- vs reduced-dose groups (P = .13).
The rate of re-treatment was significantly lower in the standard- vs reduced-dose group (0.17 vs 0.38 episodes per year, respectively; P = .039).
There was no difference in proportion of patients recovering their ADAMTS13 level into the normal range (CR, ≥60%) between the standard-dose (18 [75%] of 24) vs reduced-dose (16 [84.2%] of 19) vs intermediate-dose (12 [70.6%] of 17) groups (P = .61). Furthermore, there was no difference in time to ADAMTS13 recovery between standard-dose (median, 1 month; range, <1 to 5 months) vs reduced-dose (median, 2 months; range, <1 to 4 months) vs intermediate-dose (median, 1 month; range <1 to 3 months) groups (P = .69) (Table 3). Of patients in the other group, 14 (87.5%) of 16 had a CR at a median time to recovery of 2 months (range, <1 to 4 months).
Relapse rates
Of the 76 patient episodes, there were only 3 relapses (incidence, 3.9%) over a median follow-up period of 15 months (range, 1-141 months), occurring at 9 months, 10 months, and 32 months after reduced-dose rituximab. All had laboratory evidence of TTP with the presence of microangiopathic hemolytic anemia on blood film and elevated lactate dehydrogenase, which were associated with ADAMTS13 activity levels <5%. Two of these relapse episodes were subacute, occurring in the same patient who was asymptomatic with platelet count >100 × 109/L. The third relapse episode was associated with a worsening of pre-existing neurologic symptoms in a patient who had previously had a left middle cerebral artery infarct 4 years before his initial TTP diagnosis; his platelet count was 96 × 109/L. All 3 relapse episodes were treated with PEX. This relapse rate (3.9%) was markedly lower than the proportion of relapses (17.3%) reported via the United Kingdom TTP Registry (for all United Kingdom centers) from 2009 to 2016.
Requirement for more than 1 course of rituximab prophylaxis
Re-treatment with rituximab occurred in 38 (50%) of 76 patient episodes at a median of 17.5 months (range, 9-112 months) after the initial prophylactic dose. Thirty-five (92.1%) of 38 were re-treatment episodes with prophylaxis as a result of a measured decrease in ADAMTS13 levels to ≤15%. In the remaining 3 (7.9%) of 38 episodes, patients were re-treated with rituximab as part of treatment of either an acute (n = 1) or subacute (n = 2) TTP relapse.
Overall 20 (44.4%) of 45 patients received a prophylactic course on 2 or more occasions: 12 patients on 2 occasions, 5 patients on 3 occasions, 2 patients on 4 occasions, and 1 patient on 5 occasions. Eight patients were treated on 3 or more occasions (Table 4). Of these 20 multiply-treated patients, 16 (80%) were female and 7 (35%) were Afro-Caribbean. For the patients treated on 3 or more occasions, there was no evidence of reduced efficacy of rituximab prophylaxis, evidenced by no apparent reduction in treatment-free survival after the second or third treatment episodes compared with the first episode (Table 4). Furthermore, there was no loss of response, and repeated dosing and normalization of ADAMTS 13 activity levels were achieved. Importantly, no patient developed hypogammaglobulinemia despite repeated dosing.
Patients receiving rituximab prophylaxis on 3 or more occasions
| Patient . | Age at episode (y) . | Sex . | Ethnic group . | Treatment-free survival (mo) . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| After first episode . | After second episode . | After third episode . | After fourth episode . | After fifth episode . | |||||||||
| 1 | 46-48 | F | SE | 12 | R | 14 | I | 6 | I* | ||||
| 2 | 54-57 | F | AC | 10 | R | 17 | S | 10 | I* | ||||
| 3 | 54-56 | F | AC | 11 | S | 12 | S | 71 | S* | ||||
| 4 | 64-66 | F | AC | 12 | R | 11 | R | 10 | I* | ||||
| 5 | 25-30 | M | W | 38 | S | 25 | R | 3 | I* | ||||
| 6 | 41-47 | F | AC | 14 | S | 9 | R | 10 | R | 3 | S* | ||
| 7 | 31-39 | F | W | 29 | S | 30 | S | 31 | R | 12 | I* | ||
| 8 | 24-29 | F | AC | 14 | S | 13 | R | 13 | R | 12 | R | 9 | I* |
| Patient . | Age at episode (y) . | Sex . | Ethnic group . | Treatment-free survival (mo) . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| After first episode . | After second episode . | After third episode . | After fourth episode . | After fifth episode . | |||||||||
| 1 | 46-48 | F | SE | 12 | R | 14 | I | 6 | I* | ||||
| 2 | 54-57 | F | AC | 10 | R | 17 | S | 10 | I* | ||||
| 3 | 54-56 | F | AC | 11 | S | 12 | S | 71 | S* | ||||
| 4 | 64-66 | F | AC | 12 | R | 11 | R | 10 | I* | ||||
| 5 | 25-30 | M | W | 38 | S | 25 | R | 3 | I* | ||||
| 6 | 41-47 | F | AC | 14 | S | 9 | R | 10 | R | 3 | S* | ||
| 7 | 31-39 | F | W | 29 | S | 30 | S | 31 | R | 12 | I* | ||
| 8 | 24-29 | F | AC | 14 | S | 13 | R | 13 | R | 12 | R | 9 | I* |
AC, Afro-Caribbean; F, female; I, intermediate dose; M, male; R, reduced dose; S, standard dose; SE, Southeast Asian; W, white.
Currently in remission, not requiring re-treatment.
Rituximab dosing and need for re-treatment
There was no significant difference in the proportions of patients requiring re-treatment in the standard-dose group (12 [50%] of 24) vs reduced-dose (14 [73.7%] of 19) groups (P = .13, Fisher’s exact test). Only 3 (17.6%) of 17 patients required re-treatment in the intermediate-dose group; however, follow-up was considerably shorter: 10 months (range, 3-20 months) compared with the standard- (17.5 months [range, 1-141 months] and reduced-dose (25 months [range, 9-43 months]) groups. Ten (62.5%) of 16 patients required re-treatment in the other group at a median of 21 months follow-up (range, 3-112 months).
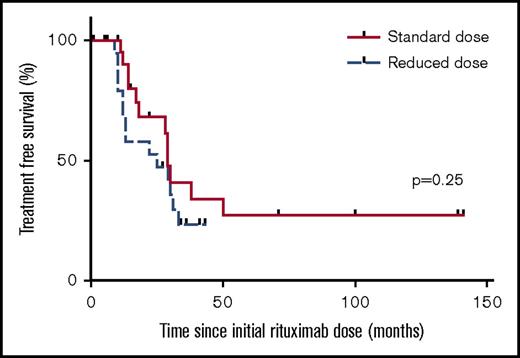
There was no difference in median treatment-free survival between standard- and reduced-dose groups (29 vs 25 months, respectively; P = .25 log-rank test) (Figure 1). However, calculating incidence rate of re-treatment based on length of follow-up in each group revealed an incidence of re-treatment episodes per year in the standard-dose group of 0.17 vs 0.38 in the reduced-dose group (P = .039).
Treatment-free survival for patients receiving standard- vs reduced-dose rituximab prophylaxis. There was no difference in median treatment-free survival between standard-dose and reduced-dose groups: 29 vs 25 months, respectively (P = .25 log-rank test).
Treatment-free survival for patients receiving standard- vs reduced-dose rituximab prophylaxis. There was no difference in median treatment-free survival between standard-dose and reduced-dose groups: 29 vs 25 months, respectively (P = .25 log-rank test).
AEs
AEs were recorded in 23 (30.3%) of 76 patient episodes, with the majority (15 [65.2%] of 23) being infusional reactions, most commonly associated with the first infusion. In all but 2 patients, these were mild and were settled with administration of hydrocortisone and chlorpheniramine. In 2 episodes that occurred in the same patient (who had received 375 mg/m2 for 3 doses for 1 course and 375 mg/m2 for 2 doses for another course), the reactions were severe: the first was a severe allergic reaction and syncope and the second was associated with tongue swelling. Of the remaining 8 (noninfusional) reactions, only 1 was severe: a patient developed severe acute serum sickness the day after a second rituximab infusion requiring treatment with steroids. This patient had been given 500 mg rituximab for 2 doses. Both patients having severe reactions (one infusional, one noninfusional) were subsequently found to have developed a human antichimeric antibody against rituximab. The remaining 7 noninfusional AEs included 3 episodes of joint pain after infusion and 1 episode of mild neutropenia, which resolved spontaneously after 1 week and was not associated with any infections. The 3 remaining patients had mild flu-like symptoms and/or general malaise. There were no episodes of hepatitis B reactivation or significant episodes of abnormal liver function tests detected after infusion. There was no evidence of hypogammaglobulinemia in patients, including those re-treated with rituximab. Furthermore, no increase in infections was noted on follow-up.
CD19 recovery after rituximab prophylaxis and CD19 level at re-treatment
CD19 recovery data were available for 62 patient episodes. Of these, 35 (56.5%) had documented CD19 recovery at a median of 11 months after the first dose of rituximab prophylaxis (range, 3 to 17 months). In the remaining 27 patient episodes, no full CD19 recovery had occurred at time of follow-up in 16 patients, and no recovery data were available for 11 patients. Of the 35 patient episodes requiring re-treatment with rituximab, 31 (88.6%) had CD19 levels within the normal range at time of re-treatment. Of these, the median time between CD19 recovery and re-treatment with rituximab was 7 months (range, 0-28 months).
Discussion
This retrospective cohort study details what is, to the best of our knowledge, the largest series of prophylactic rituximab use in TTP comprising a total of 76 patient episodes across 6 United Kingdom centers. Of these, 24 were given 4 doses (one dose per week for 4 weeks) of standard-dose rituximab (375 mg/m2), 19 were given a reduced dose (200 mg), and 17 were given an intermediate dose (500 mg). Normalization of ADAMTS13 levels into the normal range (≥60%) occurred in 78.9% of the patients, and 92.1% had an increase in ADAMTS13 to at least 30% (range, 30% to 118%) at a median of 1 month after the first rituximab dose. There was no difference in either rate of ADAMTS13 recovery or time to recovery across the 3 rituximab dose groups. Over a median of 15 months follow-up, there were only 3 episodes of TTP relapse (2 of these subacute), all occurring in 2 patients who had been given prophylaxis at a reduced dose but in whom ADAMTS13 activity could not be used as a reliable monitor.
Further prophylactic rituximab was given in 50% of the patients at a median of 17.5 months (range, 9-112 months) after the initial prophylactic treatment episode. There was no significant difference in proportions of patients requiring re-treatment (50% vs 73.7%) or treatment-free survival (29 vs 25 months) in the standard- vs reduced-dose groups. However, calculation of incidence rate of re-treatment revealed that patients with reduced dose have a re-treatment rate more than double that of the standard-dose patients (0.38 vs 0.17 re-treatment episodes per year, respectively). The proportion of re-treated intermediate-dose patients was low (17.6%), but median follow-up was short relative to that of the other 2 treatment groups (10 months), making comparison difficult. However, this may provide a compromise in elective rituximab dosing: less therapy but attainment of a more prolonged remission between treatments. It is difficult to make any definite conclusions regarding the heterogeneous group of patients (16 episodes) who received variable doses of rituximab; however, even in this group, response rates were high (87.5%), and rate of re-treatment episodes (0.29 per year) was comparable to that of other treatment groups. This suggests that rituximab prophylaxis is frequently associated with responses, irrespective of dose.
The findings presented in this study are consistent with those previously presented by our group and others,14,15 and they indicate significant benefit of rituximab prophylaxis against acute TTP relapse in patients having a documented reduction in ADAMTS13 levels from a normal baseline. Although there is no strict threshold for considering prophylaxis, we feel the cutoff of ADAMTS13 ≤15% is appropriate and identifies patients at high risk of acute relapse. Prophylaxis is effective in this group, as evidenced by the significant reduction of acute relapses (incidence 3.9%), which compares very favorably with historical rates of TTP relapse of 20% to 50%.3-6 The majority of prophylactic rituximab episodes were given between 2009 and 2016; national TTP relapse rates reported to the United Kingdom TTP Registry for this 7-year period were 17.3%. Although the national TTP relapse rate may not be directly comparable to that seen with the 6 centers included in this study, it should result in further reductions in relapse rates, given the results with prophylactic therapy.
We are not aware of any other case series comparing dosing regimens in the setting of TTP prophylaxis. The difference in re-treatment incidence rate between the reduced- and standard-dose regimens is interesting, and within the limitations of a retrospective study, it suggests that patients given reduced-dose rituximab are likely to require more frequent re-treatment than those given the standard dose. However, initial response after treatment is comparable, as evidenced by similar rates and time to normalization of ADAMTS13 in the 2 groups. Intermediate-dose rituximab seems to have an initial response rate similar to that of other regimens, but only additional follow-up will allow comparison of re-treatment rates.
Our data suggest that re-treatment with rituximab is safe and efficacious: 20 patients (44.4%) received prophylaxis on 2 or more occasions, and 8 patients received prophylaxis on 3 or more occasions. Of these 8 patients (28 episodes), there was no indication of shorter treatment-free survival after multiple episodes of rituximab prophylaxis. This finding is important because these patients would be at high risk of multiple acute relapses were it not for the use of prophylaxis. Afro-Caribbean patients in particular are more likely to need repeated rituximab doses, and this group comprised 36.8% of re-treated patients. The availability of effective prophylaxis highlights the importance of regular ADAMTS13 monitoring in the outpatient setting, with these higher-risk patients requiring more frequent monitoring.
Measurement of CD19 levels following prophylaxis revealed that recovery occurred a median of 11 months after the first dose of rituximab amounting to 56.5% of the patients. However 88.6% of patients requiring re-treatment had CD19 levels within the normal range at the time of re-treatment based on reduced ADAMTS 13 activity, which suggests that there was no temporal association between these parameters. CD19 recovery and time to re-treatment had a median of 7 months. This suggests that in most (but not all) cases, CD19 recovery is a prerequisite for TTP relapse but cannot be used as a marker for additional therapy. In other autoimmune disorders such as rheumatoid arthritis, relapse is typically seen with B-cell reconstitution.23 However, we have not found this association in TTP, and CD19 levels cannot be reliably used.
Rituximab prophylaxis is generally well tolerated, with the majority of AEs in our cohort (65.2%) being infusional reactions, mostly mild and typically occurring during the first infusion. However 3 treatment episodes were associated with severe reactions, including the development of a severe allergic reaction in 1 patient (2 episodes) and serum sickness in another. Both patients were notable in having developed human antichimeric antibodies. All the severe reactions occurred in patients who were given reduced-dose rituximab, and there was no clear indication that incidence of infusional reactions (or other AEs) was associated with dose. Although hepatitis B reactivation is a potential risk associated with rituximab use, we had no episodes of hepatitis B reactivation in our cohort. This is likely to be a reflection of our use of antiviral prophylaxis (lamivudine) in patients identified as being at risk of re-activation at baseline virology screening. The absence of severe infection and significant hypogammaglobulinemia (including in patients who were treated repeatedly) is reassuring, and it probably relates to the patients in our cohort not receiving other immunosuppressive agents and being clinically well away from their TTP diagnosis. The fact that rituximab was generally well tolerated, including in patients up to the age of 78 years in this study, indicates the potential for its effective use in the older age group.
Despite clear benefits in prolonging treatment-free survival, it is unlikely that rituximab will ever be licensed for the treatment of TTP, in either the acute or prophylactic setting. Access to this therapy is frequently restricted, and cost is not insignificant. However, set against the high mortality associated with an acute TTP episode (10% to 20%) and the previously documented risk of relapse, rituximab prophylaxis is a powerful therapy in managing patients with TTP in the longer term. Furthermore, upfront costs of outpatient monitoring and prophylaxis are likely to be far lower than those associated with an acute TTP inpatient admission, which can frequently last 2 to 3 weeks and require expensive PEX and the use of blood products; thus, there is likely to be a significant health economic benefit of this approach.
This study was limited by being retrospective, and therefore patients were not randomly assigned to any particular dose group. In addition, follow-up in the intermediate-dose group was too short to allow adequate comparison of re-treatment rates with those of the other 2 regimens. Despite these limitations, we feel this study highlights the efficacy of rituximab in the prophylactic setting, as well as the potential difference in re-treatment rates with different rituximab dose regimens. However, there is no indication that dose affects initial response rate, or that needing more frequent re-treatment is necessarily associated with worse outcomes, although receiving rituximab more frequently is likely to be associated with patients being exposed to potential AEs (typically infusional reactions). Therefore, we would recommend routine monitoring of ADAMTS13 in patients with TTP in remission. In patients having confirmed ADAMTS13 levels <15%, we would treat with prophylactic rituximab once per week for 4 weeks, assessing ADAMTS13 response once per month after therapy until ADAMTS13 recovery. Those patients having a PR (or no response) should be monitored more frequently than those attaining a CR. In patients who attain a CR, we recommend monitoring ADAMTS13 levels every 3 months. Although the ideal prophylactic dose remains unclear, according to our data, response rates seem consistent with low-, intermediate-, and standard-dose rituximab, but patients receiving low-dose therapy require re-treatment sooner than those receiving standard-dose therapy. There may be a role for intermediate-dose rituximab, but additional follow-up information is required. At this time, we suggest using a 500-mg vial of rituximab per infusion. A prospective study identifying dose and comparing response and AEs would now be beneficial.
Acknowledgments
The authors thank the following for assisting in collection of data: Debra Ellis, Siobhan Mc Guckin, and Chiara Vendramin (Department of Haematology, University College Hospital London, London, United Kingdom), Katy Langley (Haemostasis Research Unit, University College London, London, United Kingdom), Katrina Fordwor (University Hospital Birmingham National Health Service Foundation Trust, Birmingham, United Kingdom), and Clare Kay-Jones (Roald Dahl Haemostasis and Thrombosis Centre, Royal Liverpool University Hospital, Liverpool, United Kingdom).
Authorship
Contribution: J.-P.W. designed the study, collected and analyzed data, and wrote the manuscript; M.S. designed the study and wrote and revised the manuscript; M.T. and F.A. assisted with data analysis and revised the manuscript; and V.M., S.B., G.C.L., W.A.L., T.D., and Q.A.H. collected data and revised the manuscript.
Conflict-of-interest disclosure: W.A.L. has participated in an advisory board for Ablynx NV. Q.A.H. has received personal fees from Novartis and Pfizer outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: John-Paul Westwood, Department of Haematology, University College London Hospital, Third Floor West, 250 Euston Rd, London NW1 2PQ, United Kingdom; e-mail: j.westwood@ucl.ac.uk.