Abstract
Ibrutinib therapy was associated with an increased risk of bleeding in previous trials. In this systematic review and meta-analysis of published trials including patients treated with ibrutinib, the relative risk (95% confidence interval [CI]) of overall bleeding was significantly higher in ibrutinib recipients (2.72 [1.62-6.58]), but major bleeding did not show a significant difference (1.66 [0.96-2.85]). The incidences (95% CI) of major bleeding and any bleeding were 3.0 (2.3-3.7) and 20.8 (19.1-22.1) per 100 patient-years, respectively. This analysis is limited by reporting bias from variable ascertainment of bleeding and lack of allocation concealment in some studies and differing exposures between groups, leading to potential overestimation of event rates in the ibrutinib group.
Case
A 65-year-old man with chronic lymphocytic leukemia (CLL) with 17p deletion on first-line ibrutinib 420 mg daily for 8 weeks presented with a 2-week history of easy bruising and epistaxis. He was previously healthy with no personal or family history of a bleeding disorder and no regular antithrombotic drug use. Laboratory testing revealed a hemoglobin concentration of 8 g/dL, lymphocyte count of 13 × 109 /L, and normal platelet count, international normalized ratio, and activated partial thromboplastin time. What is the role of ibrutinib in this patient’s bleeding symptoms?
Introduction
Ibrutinib is an orally administered irreversible inhibitor of Bruton tyrosine kinase (BTK). BTK is an important component of the B-cell receptor signaling pathway, and it contributes to the proliferation, survival, migration, and homing of normal and malignant B cells. Ibrutinib has demonstrated substantial clinical benefit in the treatment of several B cell malignancies and has received US Food and Drug Administration approval for treatment naive and relapsed chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL),1,2 relapsed mantle cell lymphoma (MCL),3 and Waldenström macroglobulinemia.4 Additional studies are underway across a range of hematologic malignancies and solid tumors, and the approved indications for ibrutinib therapy are likely to expand in the future.
Early clinical trials of ibrutinib2,5 reported increased rates of major bleeding, including subdural hematomas, gastrointestinal bleeding, and hematuria, leading to the exclusion of patients on a vitamin K antagonist from subsequent studies.6 The true incidence, nature, and risk factors for bleeding among ibrutinib recipients remain poorly characterized owing to heterogeneity in the definitions of major bleeding used in individual studies and the lack of a priori prospective ascertainment of bleeding outcomes.
BTK plays a role in platelet signaling through GP1b and GPVI, which mediate platelet aggregation and adhesion through von Willebrand factor and collagen, respectively. However, the effect of BTK inhibition on platelet function and bleeding risk is not clear. Patients with X-linked agammaglobulinemia do not have increased bleeding despite a congenital absence of functional BTK,7 suggesting that bleeding associated with ibrutinib is more complex than BTK inhibition alone. Although the precise mechanisms for ibrutinib-associated bleeding have not been fully elucidated, published data suggest that both disease- and therapy-related platelet dysfunction may contribute to clinical bleeding. Within a phase 2 trial of single-agent ibrutinib in patients with CLL, Lipsky et al evaluated platelet function and coagulation factors at baseline and 4 weeks after initiation of therapy.8 Reduced platelet function prior to therapy, as measured by prolonged epinephrin closure time, and coagulation activity, as measured by lower factor VIII and von Willebrand factor activity, were associated with a significantly increased risk of bleeding.8 Platelet aggregation was impaired in all patients with CLL compared with healthy controls, and responses to both collagen and adenosine 5′-diphosphate agonists were reduced in ibrutinib-treated and treatment-naive patients compared with normal controls, consistent with a direct disease effect on platelet aggregation. Ibrutinib therapy was associated with further reduction in platelet aggregation with collagen while responses to adenosine 5′-diphosphate improved over time on therapy. Bleeding events appeared to decrease beyond 6 months of ibrutinib therapy, perhaps mediated by improved disease control. Bye et al9 studied the effects of ibrutinib on platelet function by comparing signaling in suspension and during adhesion to immobilized ligands. Defects in GPVI and integrin αIIbβ3 platelet signaling resulted in the formation of unstable thrombi that may contribute to bleeding. These and other findings suggest that deficiencies in several signaling pathways may ultimately contribute to ibrutininb-associated bleeding.
Methods
We conducted a systematic review of observational studies and randomized controlled trials (RCTs) with the following objectives: (1) to determine the incidence rate of overall and major bleeding events in clinical trials of ibrutinib and (2) to estimate the risk of overall and major bleeding events among patients treated with ibrutinib compared with alternative treatments. We searched MEDLINE and EMBASE for articles in any language reporting on ibrutinib, Imbruvica, or PCI-32765. Studies were excluded if they were case reports, case series, editorials, or phase 1 and dose-ranging studies or if they did not report bleeding events. Conference abstracts older than 12 months were also excluded, because they were likely to be published in medical journals, and cross-sectional studies were excluded because of an inherent high risk of recall bias. Two reviewers screened each title and abstract independently for eligibility. Disagreements were resolved by a third reviewer. The full texts of eligible papers were obtained and assessed for inclusion in duplicate. In case of overlap between articles reporting on the same cohort, we included the study with the largest cohort. The risk of bias of included studies was assessed using the Cochrane risk of bias tool10 for RCTs and methodology adapted from the American College of Chest Physicians guidelines11 for observational studies. The quality of evidence was then assessed using GRADE (Grading Recommendations Assessment and Development Evidence) criteria.12
Statistical analysis was performed using STATA 14 (StataCorp, College Station, TX). The agreement between reviewers was measured with the κ statistic. Pooled incidence rates were estimated using the approach of Guevara et al.13 Crude study-specific per-participant bleeding rates were then calculated by dividing the number of incident cases of total bleeding and major bleeding by the total number of person-months follow-up. Incidence rates were pooled after applying the Freeman-Tukey double arcsine transformation to stabilize the variances.14 Bleeding events in comparative studies were pooled (RevMan Version 5.3, The Cochrane Collaboration, 2014) with a fixed effects model, using the Mantel-Haenszel method to estimate the relative risk of bleeding. Heterogeneity was evaluated by Cochran’s Q and the I2 statistic. When heterogeneity was high, an analysis was performed using DerSimonian and Laird random effects models.
Results
The search yielded 1871 unique abstracts. There was close agreement between reviewers for title and abstract screening (κ statistic 0.86). In total, 38 full-text manuscripts and 44 conference abstracts were reviewed. Of these 82 manuscripts, 60 were excluded because they lacked data on bleeding (n = 42), did not study ibrutinib (n = 2), were dose-finding studies (n = 1) or case series (n = 1), or presented data included in another manuscript (n = 14). Twenty-two manuscripts reported on bleeding in 2152 ibrutinib recipients (Table 1). Four were RCTs, also including 759 patients on alternative therapies, 10 were phase 2 studies, 3 were prospective cohorts, and 5 were retrospective cohorts. Sixteen manuscripts were full text, and the other 6 manuscripts were abstracts presented at conferences in the last year. Fifteen studies were in patients with CLL/SLL, 4 in patients with MCL, 2 in patients with Waldenström macroglobulinemia, and 1 in a patient with follicular lymphoma.
Characteristics of included studies
| Reference . | Design . | Disease . | Indication . | Age (y), median (range) . | Sample size . | Follow-up (mo) . | Overall bleeding incidence (ibrutinib/control)* . | Major bleeding incidence (ibrutinib/control) . | Definition of major bleeding . | Risk of bias . |
|---|---|---|---|---|---|---|---|---|---|---|
| 15 | RCT | CLL/SLL | Relapsed/refractory | 67 (30-88) | 195 ibrutinib, 196 ofatumumab | 9.4 | 86/24 | 2/3 | Grade ≥3, transfused or hospitalized | Uncertain† |
| 6 | RCT | CLL/SLL | Relapsed/refractory | 64 (31-86) | 289 ibrutinib, 289 placebo | 17 | 89/42 | 11/5 | Grade ≥3 or CNS | Uncertain |
| 1 | RCT | CLL/SLL | First line | 73 (65-90) | 136 ibrutinib, 133 chlorambucil | 18.4 | — | 6/3 | Grade ≥3 or CNS | Uncertain† |
| 3 | RCT | MCL | Relapsed/refractory | 68 (IQR 13) | 139 ibrutinib, 141 temsirolimus | 20 | — | 14/9 | Grade ≥3 or CNS | High† |
| 16‡ | Phase 2 | CLL/SLL | Relapsed/refractory | 67 (55-84) | 24 | 7.5 | 13 | 1 | Grade ≥3 | High |
| 8 | Phase 2 | CLL/SLL | Both first and relapsed/refractory | 65.8 (33-85) | 86 | 24 | 47 | — | Unknown | High |
| 17 | Phase 2 | CLL/SLL | Both first and relapsed/refractory | 68 (37-84) | 132 | 36 | 81 | 10 | Unknown | High |
| 26 | Phase 2 | CLL/SLL | Both first and relapsed/refractory | 63 (35-82) | 40 | 16.8 | — | 1 | Grade ≥2 | High |
| 29‡ | Phase 2 | CLL/SLL | Relapsed/refractory | 64 | 144 | 11.5 | — | 7 | Grade 2/3 | High |
| 28 | Phase 2 | CLL/SLL | Relapsed/refractory | N = 31 >65 y | 71 | 12.5 | — | 7 | Grade ≥3 | High |
| 18‡ | Phase 2 | Follicular lymphoma | First line | 58 (32-84) | 60 | 10.2 | 13 | 0 | Unknown | High |
| 19 | Phase 2 | MCL | Relapsed/refractory | 68 (40-84) | 111 | 26.7 | 56 | 7 | Grade ≥3 | High |
| 5 | Phase 2 | MCL | Relapsed/refractory | 67 (45-86) | 50 | 16.5 | — | 1 | Requiring drug discontinuation | High |
| 15 | Phase 2 | CLL/SLL | Relapsed/refractory | 66 (37-82) | 85 | 20.9 | — | 4 | Grade ≥3 | High |
| 22 | Prospective | WM | Relapsed/refractory | 67 (47-90) | 31 | 7.7 | 5 | 0 | Unknown | High |
| 20 | Prospective | CLL/SLL | Relapsed/refractory | 66 (35-83) | 88 | 28 | 1 | — | Unknown | High |
| 4 | Prospective | WM | Relapsed/refractory | 63 (44-86) | 63 | 19.1 | — | 4 | Grade ≥2 | High |
| 24‡ | Retrospective | CLL/SLL | Both first and relapsed/refractory | 66 | 96 | 7.6 | 10 | 4 | Requiring hospitalization | High |
| 21‡ | Retrospective | CLL/SLL | Relapsed/refractory | Not stated | 124 | 6.4 | 3 | — | Requiring drug discontinuation | High |
| 23‡ | Retrospective | CLL/SLL | Relapsed/refractory | 69 | 92 | 6.8 | 30 | — | Unknown | High |
| 27‡ | Retrospective | CLL/SLL | Relapsed/refractory | 62 (36-80) | 54 | 9.1 | — | 3 | Requiring drug discontinuation | High |
| 25 | Retrospective | MCL | Relapsed/refractory | 69 (35-84) | 42 | 10.7 | 1 | — | Unknown | High |
| Reference . | Design . | Disease . | Indication . | Age (y), median (range) . | Sample size . | Follow-up (mo) . | Overall bleeding incidence (ibrutinib/control)* . | Major bleeding incidence (ibrutinib/control) . | Definition of major bleeding . | Risk of bias . |
|---|---|---|---|---|---|---|---|---|---|---|
| 15 | RCT | CLL/SLL | Relapsed/refractory | 67 (30-88) | 195 ibrutinib, 196 ofatumumab | 9.4 | 86/24 | 2/3 | Grade ≥3, transfused or hospitalized | Uncertain† |
| 6 | RCT | CLL/SLL | Relapsed/refractory | 64 (31-86) | 289 ibrutinib, 289 placebo | 17 | 89/42 | 11/5 | Grade ≥3 or CNS | Uncertain |
| 1 | RCT | CLL/SLL | First line | 73 (65-90) | 136 ibrutinib, 133 chlorambucil | 18.4 | — | 6/3 | Grade ≥3 or CNS | Uncertain† |
| 3 | RCT | MCL | Relapsed/refractory | 68 (IQR 13) | 139 ibrutinib, 141 temsirolimus | 20 | — | 14/9 | Grade ≥3 or CNS | High† |
| 16‡ | Phase 2 | CLL/SLL | Relapsed/refractory | 67 (55-84) | 24 | 7.5 | 13 | 1 | Grade ≥3 | High |
| 8 | Phase 2 | CLL/SLL | Both first and relapsed/refractory | 65.8 (33-85) | 86 | 24 | 47 | — | Unknown | High |
| 17 | Phase 2 | CLL/SLL | Both first and relapsed/refractory | 68 (37-84) | 132 | 36 | 81 | 10 | Unknown | High |
| 26 | Phase 2 | CLL/SLL | Both first and relapsed/refractory | 63 (35-82) | 40 | 16.8 | — | 1 | Grade ≥2 | High |
| 29‡ | Phase 2 | CLL/SLL | Relapsed/refractory | 64 | 144 | 11.5 | — | 7 | Grade 2/3 | High |
| 28 | Phase 2 | CLL/SLL | Relapsed/refractory | N = 31 >65 y | 71 | 12.5 | — | 7 | Grade ≥3 | High |
| 18‡ | Phase 2 | Follicular lymphoma | First line | 58 (32-84) | 60 | 10.2 | 13 | 0 | Unknown | High |
| 19 | Phase 2 | MCL | Relapsed/refractory | 68 (40-84) | 111 | 26.7 | 56 | 7 | Grade ≥3 | High |
| 5 | Phase 2 | MCL | Relapsed/refractory | 67 (45-86) | 50 | 16.5 | — | 1 | Requiring drug discontinuation | High |
| 15 | Phase 2 | CLL/SLL | Relapsed/refractory | 66 (37-82) | 85 | 20.9 | — | 4 | Grade ≥3 | High |
| 22 | Prospective | WM | Relapsed/refractory | 67 (47-90) | 31 | 7.7 | 5 | 0 | Unknown | High |
| 20 | Prospective | CLL/SLL | Relapsed/refractory | 66 (35-83) | 88 | 28 | 1 | — | Unknown | High |
| 4 | Prospective | WM | Relapsed/refractory | 63 (44-86) | 63 | 19.1 | — | 4 | Grade ≥2 | High |
| 24‡ | Retrospective | CLL/SLL | Both first and relapsed/refractory | 66 | 96 | 7.6 | 10 | 4 | Requiring hospitalization | High |
| 21‡ | Retrospective | CLL/SLL | Relapsed/refractory | Not stated | 124 | 6.4 | 3 | — | Requiring drug discontinuation | High |
| 23‡ | Retrospective | CLL/SLL | Relapsed/refractory | 69 | 92 | 6.8 | 30 | — | Unknown | High |
| 27‡ | Retrospective | CLL/SLL | Relapsed/refractory | 62 (36-80) | 54 | 9.1 | — | 3 | Requiring drug discontinuation | High |
| 25 | Retrospective | MCL | Relapsed/refractory | 69 (35-84) | 42 | 10.7 | 1 | — | Unknown | High |
–, Not available; CNS, central nervous system; IQR, interquartile range; WM, Waldenström macroglobulinemia.
If only major bleeding is reported, overall bleeding is considered not available.
Open label.
Conference abstracts.
Overall bleeding
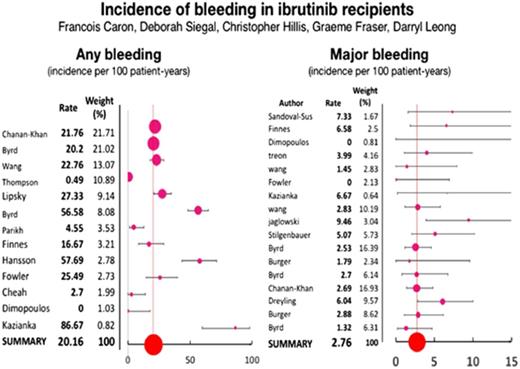
Thirteen articles reported on the incidence of overall bleeding6,8,15-25 with a pooled annual incidence of any bleeding of 20.8 per 100 patient-years (95% confidence interval [CI], 19.1-22.1) in ibrutinib-treated patients. Two RCTs6,15 compared the incidence of overall bleeding between ibrutinib and an alternative therapy (Figure 1). The pooled incidence of overall bleeding in patients receiving alternative treatments was 11.6 per 100 patient-years (95% CI, 9.1-14.4). The pooled relative risk of any bleeding with ibrutinib use was 2.72 (95% CI, 1.62-4.58; P = .0002). The I2 statistic for heterogeneity was 75%, and the heterogeneity χ2 was 3.97 (P = .05). The random effects model was used because of the significant heterogeneity of studies.
Forest plot of relative risk of overall bleeding in RCTs of ibrutinib. M-H, Mantel-Haenszel.
Forest plot of relative risk of overall bleeding in RCTs of ibrutinib. M-H, Mantel-Haenszel.
Major bleeding
Seventeen studies1-6,15-19,22,24,26-29 specifically described major bleeding, with a pooled incidence of 2.76 (95% CI, 2.07-3.53) per 100 patient-years in patients receiving ibrutinib. Major bleeding was classified according to the Common Terminology Criteria for Adverse Events and included the following: (1) grade ≥3 (n = 8),1-3,15,16,28,30,31 (2) grade ≥2 (n = 3),4,26,29 (3) reason for drug discontinuation (n = 2),5,27 and (4) bleeding requiring hospitalization (n = 1).24 In addition, 3 studies did not report a definition of major bleeding; one of these studies17 reported 10 events, including 1 resulting in death and 3 leading to drug discontinuation. In the 4 RCTs reporting on major bleeding,1,3,6,15 the pooled incidence in patients receiving alternative treatments was 1.9 (95% CI, 1.1-2.8) per 100 patient-years. The relative risk of major bleeding in ibrutinib recipients as compared with recipients of an alternative therapy was 1.66 (95% CI, 0.96-2.85; P = .07) using a fixed effects model (Figure 2). The random effects model yielded similar results. The I2 statistic for heterogeneity was 0%, and the heterogeneity χ2 was 1.35 (P = .72).
Forest plot of relative risk of major bleeding in RCTs of ibrutinib. M-H, Mantel-Haenszel.
Forest plot of relative risk of major bleeding in RCTs of ibrutinib. M-H, Mantel-Haenszel.
Discussion
This study provides moderate-quality evidence that ibrutinib increases overall bleeding compared with alternative treatments. The observational studies were judged to be at high risk of bias because of the absence of a comparator group. The definitions of major bleeding showed variability among the studies. While most studies used grade ≥3 to define major bleeding events, there was heterogeneity in those definitions and even more in the definition and reporting of minor bleeding. The quality of evidence for RCTs was downgraded due to heterogeneity between studies in reporting of bleeding outcomes. Our meta-analysis was likely underpowered with regards to major bleeding complications, so a difference in the risk of major bleeding between ibrutinib and other treatments cannot be excluded. Some limitations of our analysis should be noted. Bleeding events may have been underestimated in some studies, as they were not captured a priori as study outcomes with clear definitions, systematic data collection, or adjudication processes reported. Lack of concealment of treatment allocation may have led to reporting bias in some trials. Few details were provided regarding factors that may have contributed to bleeding events such as the use of antithrombotic therapy or concurrent severe thrombocytopenia. Finally, in the 4 RCTs, patients were exposed to ibrutinib for longer periods than to the control treatment. This could lead to an overestimation of ibrutinib-related adverse events. Dreyling et al3 performed an exposure-adjusted analysis and revealed bleeding rates of 0.8 per 100 patient-months in the ibrutinib group and 1.1 per 100 patient-months in the temsirolimus group. Exposure-adjusted incidence rates were not reported by the other authors. As a sensitivity analysis, we calculated incidence rates based on the duration of exposure (Table 2). This analysis removes any difference in rates of major bleeding between groups.
Incidence of bleeding adjusted for exposure
| Reference . | Median exposure (mo) . | Overall bleeding incidence* . | Major bleeding incidence* . | |||
|---|---|---|---|---|---|---|
| Ibrutinib . | Control . | Ibrutinib . | Control . | Ibrutinib . | Control . | |
| 15 | 8.4 | 5.3 | 63.0 | 27.6 | 1.4 | 3.5 |
| 1 | 17.4 | 7.1 | — | — | 3.0 | 3.8 |
| 3 | 14.4 | 3 | — | — | 8.4 | 25.9 |
| 6 | 14.7 | 12.8 | 25.1 | 13.6 | 3.1 | 1.7 |
| Reference . | Median exposure (mo) . | Overall bleeding incidence* . | Major bleeding incidence* . | |||
|---|---|---|---|---|---|---|
| Ibrutinib . | Control . | Ibrutinib . | Control . | Ibrutinib . | Control . | |
| 15 | 8.4 | 5.3 | 63.0 | 27.6 | 1.4 | 3.5 |
| 1 | 17.4 | 7.1 | — | — | 3.0 | 3.8 |
| 3 | 14.4 | 3 | — | — | 8.4 | 25.9 |
| 6 | 14.7 | 12.8 | 25.1 | 13.6 | 3.1 | 1.7 |
–, Not available.
Incidence is reported per 100 person-years of exposure.
The incidence rates of overall and major bleeding observed with ibrutinib use are high enough to warrant concern. Although not directly comparable, major bleeding complications in patients receiving anticoagulant therapy for atrial fibrillation occur at rates of 2.1 to 3.6 per 100 patient-years with case fatality rates of 7% to 14% in clinical trials.32-36 With the expected rise in ibrutinib use for the treatment of B-cell lymphoproliferative diseases, bleeding events will likely become an increasingly significant clinical concern, especially among elderly patients requiring concurrent antithrombotic therapy who were underrepresented or excluded from clinical trials.1-5 An increased rate of atrial fibrillation has been observed in clinical trials of ibrutinib.37,38 While there is net benefit from anticoagulation in individuals from the general population with atrial fibrillation and additional stroke risk factors, the increased bleeding risk in ibrutinib-treated patients may alter the risk-benefit ratio of anticoagulant therapy for the prevention of stroke and systemic embolism. To our knowledge, there is no evidence regarding the stroke risk in ibrutinib-treated patients with atrial fibrillation or whether widely used clinical prediction models for stroke, such as CHADS2 or CHADS2Vasc, or bleeding, such as HAS-BLED or ATRIA, are helpful in this setting.39-41 Patients with an indication for warfarin were excluded from the later trials after high rates of bleeding were noticed in earlier studies. Until further data identify the risk factors for bleeding, the decision to initiate anticoagulant therapy should be individualized based on a discussion of patient values and preferences and considering the available evidence regarding the benefits and risks of therapy.
Another relevant clinical scenario is the patient requiring dual antiplatelet therapy (DAPT) after percutaneous coronary intervention with stent placement. Following drug-eluting coronary stent implantation, a minimum of 12 months of DAPT with aspirin and clopidogrel/ticagrelor is preferred for the prevention of late stent thrombosis, which confers a 20% to 45% case-fatality rate. Bare metal stents struts are covered more rapidly by endothelium so are associated with a lower risk of late-stent thrombosis. A minimum of 2 to 4 weeks of DAPT is considered important after bare metal coronary stent implantation, making it a more attractive alternative in the patient with an indication for percutaneous coronary intervention.42
In the event of clinically relevant bleeding, the ibrutinib product monograph43 advises a dose reduction by 1 tablet, although the effect of this approach on hematologic disease and the risk of subsequent bleeding events are unknown. The indications and dosages of antithrombotic therapies, nonsteroidal anti-inflammatory drugs, and herbal supplements should be reviewed. Drug interactions with inhibitors of CYP3A4 should be explored, as they could increase the concentration of ibrutinib. In cases of major bleeding, supportive therapy should be promptly initiated, and reversible contributing factors addressed. Ibrutinib should be withheld until resolution of the bleeding; the decision to resume ibrutinib will depend on the ongoing bleeding risk and the status of the underlying malignancy. There is a paucity of data regarding the bleeding risk of invasive procedures or surgeries in patients receiving ibrutinib. The product monograph advises temporary interruption of ibrutinib 7 days prior to an invasive procedure.
This review points to the importance of developing standardized reporting of cardiovascular outcomes for oncology studies. Definitions for bleeding events have been developed by the International society of thrombosis and hemostasis44 to be used in studies of products that interfere with coagulation. The use of such definitions would decrease the interstudy variability outcome reporting. Our analysis also revealed that reporting of treatment-emergent adverse events is a source of bias in studies with large difference in exposure time between groups. Intention-to-treat analysis or exposure-adjusted incidences can help to minimize this effect. Bleeding has emerged as an important adverse effect of ibrutinib. Recommendations regarding the approach to bleeding in ibrutinib recipients are largely empiric at present, and more data on the risk of bleeding, bleeding risk factors, and the risks of discontinuing ibrutinib are needed to permit evidence-guided recommendations.
Acknowledgments
D.P.L. is supported by the E. J. Moran Campbell Career Award from McMaster University and by the Hamilton Health Sciences RFA Strategic Initiative Program. C.H. is supported by a fellowship from the Department of Oncology, McMaster University. D.S. is supported by a fellowship award from the Canadian Institutes of Health Research.
Authorship
Contribution: F.C., D.P.L., and D.S. contributed to data extraction; F.C. and D.P.L. analyzed the data; D.S. and F.C. assessed the risk of bias; and all authors contributed to writing the paper.
Conflict-of-interest disclosure: D.P.L. has received honoraria from Janssen Pharmaceuticals. G.F. has received honoraria and research funding from Janssen Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: François Caron, David Braley Building, Hamilton General Hospital, 237 Barton St E, Hamilton, ON L8L 2X2, Canada; e-mail: francois.caron@phri.ca.
References
Author notes
F.C. and D.P.L. contributed equally to this study.