Key Points
In vivo CRISPR screening allows efficient evaluation of DLBCL drivers, showing cooperation of Kmt2d/Trp53 losses in B-cell lymphomagenesis.
Combined Kmt2d and Trp53 inactivation induces Yap1 overexpression in B-cell lymphomas and Yap1 inhibition suppresses their tumorigenesis.
Visual Abstract
Although recent genetic studies have identified numerous genetic alterations in diffuse large B-cell lymphoma (DLBCL), their biological relevance remains elusive. Here, we performed in vivo CRISPR loss-of-function screening targeting 86 genes recurrently altered in DLBCL to examine oncogenicity of single-guide RNA (sgRNA)–targeted genes, association between genotype and lineage, occurrence of second-hit alterations, and cooperability among sgRNA-targeted genes and second-hit alterations. Transplantation of the CRISPR library–transduced hematopoietic stem/progenitor cells induces various hematologic malignancies, including B-cell lymphomas in mice. Enrichment analysis of sgRNA-targeted genes demonstrates significant overrepresentation of Kmt2d, Pax5, and Trp53 in B-cell lymphomas. Whole-exome sequencing identifies recurrent second-hit driver alterations, showing significant enrichment of Trp53 alterations in sgKmt2d-targeted B-cell lymphomas. Importantly, KMT2D and TP53 mutations are found to be the most prevalent co-occurring combination in human DLBCL, which is more prominent in relapsed/refractory DLBCL. Moreover, this combination confers significantly worse prognosis independent of clinical factors. Transcriptomic sequencing identifies overexpression of Yap1, the Hippo pathway component, in double sgKmt2d-targeted/Trp53-altered B-cell lymphomas. Furthermore, chromatin accessibility analysis demonstrates enrichment of transcriptional enhanced associate domain 1 binding motifs in regions that gained accessibility and increased expression of their nearest genes in these B-cell lymphomas. Most importantly, genetic and pharmacological inhibition of YAP1 suppresses in vitro cell proliferation and in vivo tumor growth of a human KMT2D/TP53-altered DLBCL cell line and prolongs survival of mice transplanted with double sgKmt2d-targeted/Trp53-altered B-cell lymphoma cells. Our findings demonstrate the utility of in vivo CRISPR screening to integrate human cancer genomics with mouse modeling and highlight the functional interplay between KMT2D and TP53 aberrations, providing insights into therapeutic strategies in DLBCL.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is a clinically, phenotypically, and genetically heterogeneous disease entity, representing 30% to 40% of non-Hodgkin lymphomas.1,2 This disease typically presents with progressive lymphadenopathy (LAD) and/or extranodal disease and is characterized by diffuse proliferation of medium or large lymphoid B cells. Despite the advanced stage at presentation in most patients, 60% to 70% can be cured with standard immunochemotherapy.3 However, patients with treatment failure after initial therapy often have a poor prognosis.
Gene expression profiling divides DLBCL into 2 distinct molecular subtypes, the germinal center (GC) B-cell–like and activated B-cell–like subtypes, which are believed to arise from different stages of lymphoid differentiation or cell of origin.4 Genetic studies using next-generation sequencing have identified many recurrent genetic alterations including mutations, copy number alterations (CNAs), and structural variations, and revealed their overall picture in DLBCL.5-10 These analyses have led to proposals of new taxonomies for DLBCL, yielding genetically defined subtypes beyond cell of origin.3,11,12 For example, the LymphGen algorithm classifies DLBCL into 7 genetic subtypes, such as MCD (MYD88 L265P and CD79B mutations), EZB (EZH2 mutation and BCL2 translocation), and A53 (TP53 mutation and aneuploidy).13
Although a long tail of recurrently mutated genes (including a few hundred genes) have been reported in DLBCL, in vivo roles in B-cell lymphomagenesis have been thoroughly explored for a limited number of genes.14-23 In addition, these long tail genes include many targets of aberrant somatic hypermutations, such as PIM1 and BCL2, some of which are considered to be functionally irrelevant.24 Therefore, whether and how many of these recurrently mutated genes function as a driver or not, remain elusive. In addition, although several driver mutations are required to cooperate to induce B-cell lymphomagenesis, such cooperability have not been well investigated.
The clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR–associated protein 9 (Cas9) technology is a robust and efficient tool for targeted genome modification.25 Functional screening using this technology enables the simultaneous investigation of the effect of genetic perturbation on cellular proliferation, therapeutic vulnerability, and expression profiles for thousands of genes in vitro in various malignancies.26-28 In vivo, combinatorial CRISPR-mediated genome editing targeting ∼10 genes has been applied to rapid engineering cancer models and evaluating mutational cooperability in leukemia.29,30 More recently, an in vivo CRISPR screening approach targeting tens to hundreds of genes has been developed to elucidate the tumorigenic potential of loss-of-function alterations in leukemia and solid cancers.31,32
Here, through a bone marrow transplantation model, we performed in vivo CRISPR loss-of-function screening targeting 86 recurrently altered genes in DLBCL to examine the tumorigenic capacity of single-guide RNA (sgRNA)–targeted genes. In addition, we investigated genotype-phenotype association, occurrence of second-hit alterations, and cooperability between sgRNA-targeted genes and second-hit alterations in murine lymphoid neoplasms.
Methods
In vivo CRISPR loss-of-function screening
To construct the CRISPR library, 86 genes in which loss-of-function alterations (mutations and deletions) are recurrently (>5%) observed in DLBCL5-10 were chosen. For each gene, 12 sgRNAs were designed using the genetic pertubation platform sgRNA Design tool.33 Cloning of a custom CRISPR library has been described previously.34
Hematopoietic stem/progenitor cells (HSPCs; Lin−Sca-1+c-Kit+) collected from constitutively Cas9-expressing mice were transduced with either of the concentrated lentiviral CRISPR libraries at a multiplicity of infection of 0.5 using RetroNectin (Takara Bio). Then, red fluorescent protein positive cells were isolated and IV injected into lethally irradiated C57BL/6J mice at a dose of 5 × 103 cells together with 1 × 105 competitor cells. Details of additional experiments are described in supplemental Methods.
Results
Development of a variety of murine hematologic malignancies in CRISPR loss-of-function screening targeting recurrently mutated genes in DLBCL
Because many driver candidates mutated with various frequencies have been reported in DLBCL, we constructed a custom lentiviral CRISPR library (pool A) targeting 86 genes in which loss-of-function alterations are recurrently (>5%) observed in DLBCL, including aberrant somatic hypermutations targets5-10 (Figure 1A; supplemental Figure 1A; supplemental Tables 1 and 2). In addition, we constructed another CRISPR library (pool B) by excluding 4 tumor suppressor genes. These sgRNA-targeted genes also covered most driver candidates of other precursor and mature lymphoid neoplasms, such as 64.7% of those observed in B-cell acute lymphoblastic leukemia/lymphoblastic lymphoma (B-ALL/LBL)35 and 86.2% of those observed in peripheral T-cell lymphoma, not otherwise specified36 (Figure 1B; supplemental Table 1).
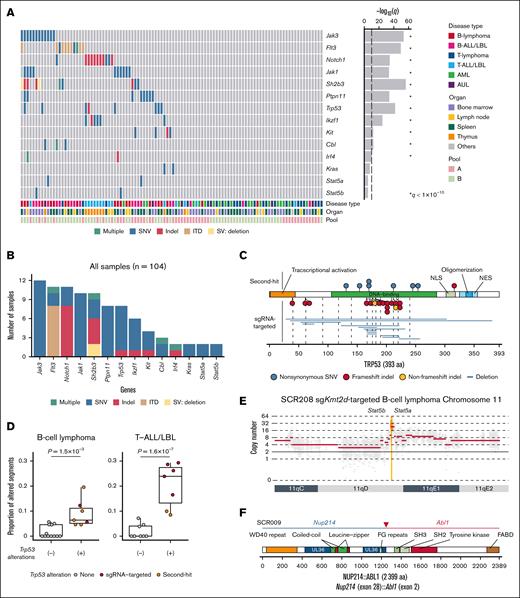
CRISPR loss-of-function screening of recurrently mutated genes in DLBCL. (A) Schematic representation of CRISPR loss-of-function screening targeting 86 recurrently mutated genes in DLBCL. (B) Overlap of sgRNA-targeted genes with recurrently altered TSGs in B-ALL/LBL, and PTCL, NOS. Because of interspecies differences, 86 murine genes in the CRISPR library correspond to 84 orthologous human genes. (C) Survival curves of recipient mice transplanted with HSPCs transduced with mock plasmid (n = 6) or the CRISPR library (n = 199). Log-rank test. (D) Distribution of hematologic malignancies in recipient mice (n = 132) according to the Bethesda proposals. (E) Landscape of significantly recurrent (q < 0.01) sgRNA-mediated disruption in the entire cohort (n = 104). Disease type, involved organ, and CRISPR library pool (bottom) as well as q values (right) are shown. (F-K) Number of samples harboring sgRNAs for each gene in the entire cohort (F; n = 104), B-cell lymphoma (G; n = 20), B-ALL/LBL (H; n = 19), T-cell lymphoma (I; n = 24), T-ALL/LBL (J; n = 15), and AML (K; n = 20). Significantly recurrent genes (q < 0.01) are colored and marked with asterisks. AML, acute myeloid leukemia; AUL, acute undifferentiated leukemia; NGS, next-generation sequencing; NK-lymphoma, natural killer-cell lymphoma; PTCL NOS, peripheral T-cell lymphoma, not otherwise specified; TSGs, tumor suppressor genes.
CRISPR loss-of-function screening of recurrently mutated genes in DLBCL. (A) Schematic representation of CRISPR loss-of-function screening targeting 86 recurrently mutated genes in DLBCL. (B) Overlap of sgRNA-targeted genes with recurrently altered TSGs in B-ALL/LBL, and PTCL, NOS. Because of interspecies differences, 86 murine genes in the CRISPR library correspond to 84 orthologous human genes. (C) Survival curves of recipient mice transplanted with HSPCs transduced with mock plasmid (n = 6) or the CRISPR library (n = 199). Log-rank test. (D) Distribution of hematologic malignancies in recipient mice (n = 132) according to the Bethesda proposals. (E) Landscape of significantly recurrent (q < 0.01) sgRNA-mediated disruption in the entire cohort (n = 104). Disease type, involved organ, and CRISPR library pool (bottom) as well as q values (right) are shown. (F-K) Number of samples harboring sgRNAs for each gene in the entire cohort (F; n = 104), B-cell lymphoma (G; n = 20), B-ALL/LBL (H; n = 19), T-cell lymphoma (I; n = 24), T-ALL/LBL (J; n = 15), and AML (K; n = 20). Significantly recurrent genes (q < 0.01) are colored and marked with asterisks. AML, acute myeloid leukemia; AUL, acute undifferentiated leukemia; NGS, next-generation sequencing; NK-lymphoma, natural killer-cell lymphoma; PTCL NOS, peripheral T-cell lymphoma, not otherwise specified; TSGs, tumor suppressor genes.
We introduced the CRISPR library into murine HSPCs collected from constitutively Cas9-expressing mice and transplanted them into lethally irradiated wild-type mice (Figure 1A). Irrespective of library pool and sheep red blood cell injection, 180 (90.5%) of 199 recipient mice exhibited moribund condition suggestive of hematologic malignancies, resulting in earlier death than control mice, with a median survival of 228 days (range, 37-476; Figure 1C; supplemental Figure 1B-D). Hematologic, immunophenotypic, and pathologic analyses of 132 tumors revealed that these malignancies consisted of B-cell and T-cell lymphomas (15.9% and 22.0%, respectively), B-ALL/LBL and T-ALL/LBL (14.4% and 15.2%, respectively), and acute myeloid leukemia (15.2%) according to the Bethesda criteria,37 with almost no difference between pool A and B (Figure 1D; supplemental Figure 2A-C; supplemental Table 3). In B-cell malignancies, B-cell lymphoma showed more extensive splenomegaly and LAD and exhibited immunoglobulin light chain restriction, whereas B-ALL/LBL showed leukocytosis and anemia and lacked immunoglobulin light chain expression (supplemental Figure 2D-E). In T-cell malignancies, T-cell lymphoma was generally CD4+, whereas T-ALL/LBL showed both CD4 and CD8 positivity and was mostly accompanied with thymus enlargement (supplemental Figure 2F). Acute myeloid leukemia showed leukocytosis and anemia, with increased myeloid populations (Gr-1+, Mac-1+, CD41+, CD71+, or TER-119+) in the bone marrow or peripheral blood (supplemental Figure 2G). Other tumors included those lacking clear lineage commitment (4.6%, diagnosed as acute undifferentiated leukemia), those that developed nonhematologic malignancies (5.3%), and those arising from non–library-transduced (red fluorescent protein negative) cells (6.8%), which developed later than B/T-cell malignancies (supplemental Figure 2C). These results suggest that disruption of recurrently altered genes in human DLBCL can induce various kinds of hematologic malignancies in mice.
Characterization of oncogenicity and phenotypic association of the sgRNA-targeted genes
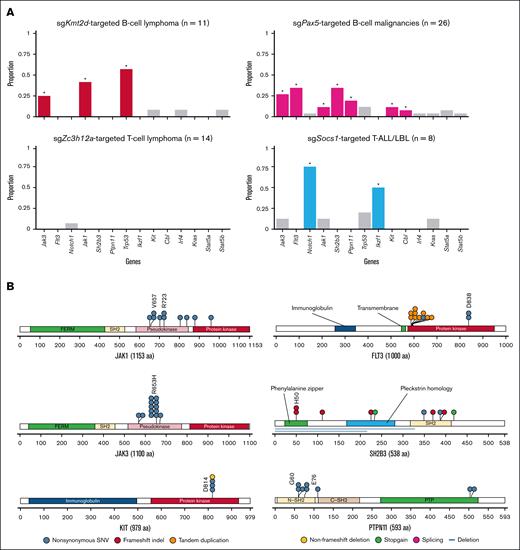
To identify sgRNA-targeted genes inducing various hematologic malignancies, we performed sgRNA sequencing of 104 tumors (supplemental Table 4). This analysis showed that at least 1 sgRNA-targeted gene was detected in all tumors, with a median of 3 (range, 1-7) genes per tumor (Figure 1E; supplemental Figure 3A). Enrichment analysis of sgRNA-targeted genes in all tumors demonstrated significant (q < 0.01) overrepresentation of 7 genes, namely Pax5, Kmt2d, Zc3h12a, Socs1, Trp53, Cdkn2a, and Irf2bp2, pointing to a stronger oncogenic capacity of these driver genes (Figure 1F). Multiple sgRNAs were detected for these recurrently targeted genes, validating the on-target effect of these sgRNAs (supplemental Figure 3B). The sgRNA-targeted genes showed strong disease type specificity (Figure 1G-K). Significant enrichment of Pax5 was observed in both B-ALL/LBL and B-cell lymphoma, whereas Kmt2d was only overrepresented in B-cell lymphoma. Most of sgZc3h12a-targeted tumors were T-cell lymphoma, whereas Socs1 was enriched in T-ALL/LBL. In contrast, Trp53 was significantly overrepresented in B-cell lymphoma and T-ALL/LBL, although sgTrp53-targeted tumors were observed in various kinds of lineage. Among them, sgKmt2d-targeted B-cell lymphoma and sgZc3h12a-targeted T-cell lymphoma showed shorter survival (supplemental Figure 3C). These observations suggest that loss-of-function of a limited number of genes results in the development of murine hematologic malignancies, which show clear genotype-phenotype associations. Particularly, Kmt2d, Pax5, and Trp53 are strongly enriched in B-cell lymphoma, consistent with human DLBCL.5-7
Second-hit alterations cooperate to induce murine hematologic malignancies
To examine the efficiency of the sgRNA-targeted disruption and explore additional somatic alterations, we performed whole-exome sequencing (WES) analysis of 104 tumors (supplemental Figure 4A). In total, we detected 1188 somatic mutations (0.33 mutations per megabase per sample), including 679 single-nucleotide variants and 509 insertions and deletions (indels), as well as 158 structural variations (including deletions and internal tandem duplications; supplemental Tables 5 and 6). B-cell lymphoma and T-ALL/LBL harbored a greater number of mutations than other disease types (supplemental Figure 4B). We validated the CRISPR/Cas9-induced indels in 72.5% of the sgRNA-targeting sites (supplemental Figure 4C). Particularly, 85.7% of sgRNAs successfully disrupted the gene in those recurrently targeted by sgRNAs, whereas as many as 33.5% of sgRNAs failed to induce indels in other genes (supplemental Figure 4D).
Enrichment analysis of second-hit alterations in all tumors demonstrated significant overrepresentation of 11 genes. These included not only genes reported as second-hit mutations in mice, such as Jak3, Notch1, Jak1, Ptpn11, Ikzf1, Kit, and Trp53,38-40 but also novel genes, such as Flt3, Sh2b3, Cbl, and Irf4 (Figure 2A-B). Although sgTrp53 was included in CRISPR library pool A, Trp53 mutations outside the sgRNA-targeting sites were recurrently observed in tumors transduced with library pool B (Figure 2C), suggesting that many Trp53 mutations occur as a second-hit alteration. We also investigated hotspot mutations in other driver genes of human lymphoid malignancies and found Stat5a (N642H), Stat5b (Q706L), and Kras (Q61H) hotspot mutations in B-cell malignancies (supplemental Figure 4E). In total, WES analysis identified additional driver mutations in 87.2% and 35.9% of B-cell and T-cell neoplasms, respectively, suggesting the strong necessity of second-hit alterations in murine lymphoid neoplasms.
Second-hit driver alterations revealed by WES in CRISPR screening. (A) Landscape of significantly recurrent second-hit somatic alterations in the entire cohort. Disease type, involved organ, and CRISPR library pool (bottom) as well as q values (right) are shown. Significantly recurrent genes (q < 1 × 10−10) are marked with asterisks. (B) Number of samples harboring second-hit alterations for each gene in the entire cohort (n = 104). (C) Type and position of Trp53 second-hit alterations in the entire cohort. (D) Proportion of copy number–altered segments in samples with and without Trp53 alterations in B-cell lymphoma (n = 19) and T-ALL/LBL (n = 15). Brunner-Munzel test. (E) Stat5a/Stat5b amplification in a B-cell lymphoma case (SCR208). (F) Nup214::Abl1 fusion in a B-ALL/LBL case (SCR009). aa, amino acids; AUL, acute undifferentiated leukemia; ITD, internal tandem dupulication; NES, nuclear export signal; NLS, nuclear localization signal; SNV, single-nucleotide variant; SV, structural variation.
Second-hit driver alterations revealed by WES in CRISPR screening. (A) Landscape of significantly recurrent second-hit somatic alterations in the entire cohort. Disease type, involved organ, and CRISPR library pool (bottom) as well as q values (right) are shown. Significantly recurrent genes (q < 1 × 10−10) are marked with asterisks. (B) Number of samples harboring second-hit alterations for each gene in the entire cohort (n = 104). (C) Type and position of Trp53 second-hit alterations in the entire cohort. (D) Proportion of copy number–altered segments in samples with and without Trp53 alterations in B-cell lymphoma (n = 19) and T-ALL/LBL (n = 15). Brunner-Munzel test. (E) Stat5a/Stat5b amplification in a B-cell lymphoma case (SCR208). (F) Nup214::Abl1 fusion in a B-ALL/LBL case (SCR009). aa, amino acids; AUL, acute undifferentiated leukemia; ITD, internal tandem dupulication; NES, nuclear export signal; NLS, nuclear localization signal; SNV, single-nucleotide variant; SV, structural variation.
We also analyzed CNAs using the WES data and found that aneuploidy frequently occurred in B-cell lymphoma and T-ALL/LBL (supplemental Figure 5A-C). Particularly, Trp53-mutated tumors commonly exhibited gross aneuploidy, regardless of whether Trp53 mutations were CRISPR/Cas9-induced indels or second-hit mutations (Figure 2D). These observations were consistent with human TP53-mutated DLBCL (the A53 subtype).6,13 Although CNAs mainly consisted of arm-level changes, focal amplifications at the 11q involving Stat5a and Stat5b were observed in 2 mice with B-cell lymphoma (Figure 2E).
To evaluate the gene expression profiles of murine hematologic neoplasms, we also performed RNA-sequencing (RNA-seq) analysis of 95 tumors (supplemental Figures 1D and 6A; supplemental Table 3). Uniform manifold approximation and projection identified different clusters associated with involved organs, disease types, and sgRNA-targeted genes (supplemental Figure 6B). Particularly, sgKmt2d-targeted B-cell lymphomas, sgPax5-targeted B-cell malignancies, sgSocs1-targeted T-ALL/LBL, and sgZc3h12a-targeted T-cell lymphomas formed distinct clusters. In addition, RNA-seq identified a Nup214::Abl1 fusion in B-ALL/LBL, which was compatible with NUP214::ABL1 fusions in human B-ALL/LBL41 (Figure 2F). Thus, a combined approach of in vivo CRISPR screening and integrative genomic profiling reveals a variety of second-hit alterations, including CNAs and fusions, occurring in murine lymphoid malignancies.
Association of second-hit alterations with disease types and sgRNA-targeted genes
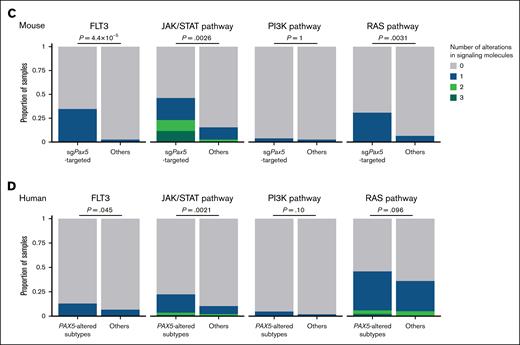
The second-hit alterations exhibited significant association with disease types and sgRNA-targeted genes (Figure 3A). Jak3 and Jak1 mutations were significantly enriched in sgKmt2d-targeted B-cell lymphoma and sgPax5-targeted B-cell malignancies. R653H mutations comprised a prominent hotspot in Jak3, and most mutations were distributed within the pseudokinase domain of Jak1, suggesting that these mutations were gain-of-function, consistent with JAK3 and JAK1 mutations in human B-ALL/LBL42 (Figure 3B). Mutations affecting Flt3, Sh2b3, Ptpn11, Kit, and Cbl were predominantly observed in sgPax5-targeted B-cell malignancies (Figure 3A). Sh2b3 and Cbl mutations were mainly consisted of nonsense, frameshift, and splice-site mutations, suggesting their loss-of-function nature (Figure 3B; supplemental Table 5). By contrast, internal tandem duplications and hotspot missense mutations at D838 were recurrently observed in Flt3. In addition, missense mutations were preferentially localized in the N-SH2 and PTP domains of the Ptpn11 gene. Furthermore, all of Kit mutations were present at a D814 hotspot. The mutational spectrums correspond to those observed in human B-cell neoplasms.42 Taken together, JAK/STAT pathway alterations (Jak1, Jak3, Stat5a, and Stat5b mutations and amplifications) were enriched in sgKmt2d-targeted B-cell lymphoma, whereas sgPax5-targeted B-cell malignancies were characterized by frequent alterations involving various signaling pathways, including FMS-like tyrosine kinase 3 (FLT3), JAK/STAT, and rat sarcoma virus (RAS) pathways (Figure 3C). The latter findings are consistent with human B-ALL/LBL data showing higher frequencies of somatic alterations belonging to the same pathways in PAX5-altered cases, although there were some differences of altered genes within the pathways between human and mice (Figure 3D).
Besides signaling pathways, Trp53 missense mutations outside the sgRNA-targeting sites were strongly enriched in sgKmt2d-targeted B-cell lymphoma, although Trp53 second-hit mutations were also observed in sgPax5-targeted B-cell malignancies and sgSocs1-targeted T-ALL/LBL (Figure 3A). Frequent mutations involving Notch1 and Ikzf1 were found in sgSocs1-targeted T-ALL/LBL (Figure 3A). C-terminal truncation was recurrently observed in Notch1, whereas loss-of-function mutations mainly consisted of Ikzf1 mutations (supplemental Figure 7A). By contrast, a smaller number of somatic mutations with lower allele frequencies were detected in sgZc3h12a-targeted tumors (supplemental Figure 7B-C). In addition, monoclonal T-cell receptor clonality estimated by RNA-seq was lower in these tumors (supplemental Figure 7D). These results suggest that sgZc3h12a-targeted tumors are caused by lymphoproliferation, consistent with previous reports.43 Taken together, these findings demonstrate cooperative relationships between specific sgRNA-targeted genes and second-hit alterations as well as their association with lineage.
Co-occurrence of KMT2D and TP53 mutations is frequent and associated with worse prognosis in human DLBCL
Significant enrichment of Kmt2d and Trp53 in sgRNA-targeted genes and Trp53 in second-hit mutations in murine B-cell lymphoma led us to investigate the functional interplay between them in B-cell lymphomagenesis. Indeed, sgKmt2d-targeted B-cell lymphoma more frequently harbored Trp53 alterations, including sgRNA-targeted disruption and second-hit mutations (Figure 4A). Particularly, second-hit mutations of Trp53 were more common in sgKmt2d-targeted B-cell lymphoma, whereas the frequency of sgRNA-targeted disruption of Trp53 was comparable between sgKmt2d-targeted B-cell lymphoma and other tumors. In sgKmt2d-targeted B-cell lymphoma, Trp53-altered cases showed similar phenotype, regardless of whether sgRNA-targeted disruption or second-hit alterations (supplemental Figures 2E and 8). In contrast, none exhibited second-hit alterations in Kmt2d in B-cell lymphomas (n = 20).
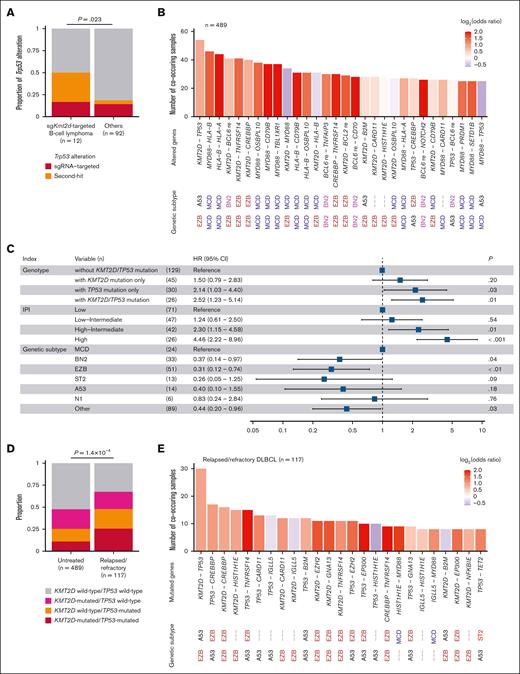
Co-occurring KMT2D and TP53 mutations are prevalent and adversely prognostic in human DLBCL. (A) Proportion of Trp53 alterations in murine sgKmt2d-targeted B-cell lymphoma (n = 12) and others (n = 92). Fisher exact test. (B) Number of co-occurring combinations of driver alterations in 489 untreated patients with DLBCL from National Cancer Institute's Center for Cancer Research (NCICCR) cohort. (C) HRs with 95% CIs for overall survival evaluated by Cox proportional hazards model incorporating presence of KMT2D, TP53, or both mutations, IPI, and genetic subtype according to the LymphGen classification in 230 untreated patients with DLBCL from NCICCR cohort. (D) Proportion of KMT2D, TP53, or both mutations in 489 untreated and 117 patients with relapsed/refractory DLBCL. (E) Number of co-occurring combinations of driver mutations in 117 patients with relapsed/refractory DLBCL. In panels B,E, genetic subtype related to each altered gene is shown according to the LymphGen classification. Bars are colored by odds ratios calculated by Fisher exact test. Aberrant somatic hypermutation target genes are removed. CI, confidence interval; HR, hazard ratio; IPI, International Prognostic Index; re, rearrangement.
Co-occurring KMT2D and TP53 mutations are prevalent and adversely prognostic in human DLBCL. (A) Proportion of Trp53 alterations in murine sgKmt2d-targeted B-cell lymphoma (n = 12) and others (n = 92). Fisher exact test. (B) Number of co-occurring combinations of driver alterations in 489 untreated patients with DLBCL from National Cancer Institute's Center for Cancer Research (NCICCR) cohort. (C) HRs with 95% CIs for overall survival evaluated by Cox proportional hazards model incorporating presence of KMT2D, TP53, or both mutations, IPI, and genetic subtype according to the LymphGen classification in 230 untreated patients with DLBCL from NCICCR cohort. (D) Proportion of KMT2D, TP53, or both mutations in 489 untreated and 117 patients with relapsed/refractory DLBCL. (E) Number of co-occurring combinations of driver mutations in 117 patients with relapsed/refractory DLBCL. In panels B,E, genetic subtype related to each altered gene is shown according to the LymphGen classification. Bars are colored by odds ratios calculated by Fisher exact test. Aberrant somatic hypermutation target genes are removed. CI, confidence interval; HR, hazard ratio; IPI, International Prognostic Index; re, rearrangement.
To investigate the relevance of the co-occurrence of KMT2D and TP53 mutations in human DLBCL, we evaluated the frequency of combinations of driver mutations using WES data of 489 untreated patients with DLBCL from the National Cancer Institute's Center for Cancer Research cohort.7 Most of frequently co-occurring mutations consisted of those characterizing the genetic subtypes according to the LymphGen classification, such as MYD88 and HLA-B mutations in MCD subtype and KMT2D and TNFRSF14 mutations in EZB subtype.13 However, importantly, KMT2D and TP53 mutations were the most frequent co-occurring combination, although these 2 genes are characteristic of different subtypes (Figure 4B). This co-occurrence was found in 54 patients (11.0%) with DLBCL.
Then, we evaluated the effect of the co-occurrence of KMT2D and TP53 mutations on prognosis in untreated DLBCL in multivariable analysis incorporating International Prognostic Index and genetic subtype according to the LymphGen classification. Notably, although individual KMT2D or TP53 mutations tended to be associated with worse prognosis, the co-occurrence of KMT2D and TP53 mutations significantly and independently predicted a shorter overall survival (Figure 4C). In addition, we compared untreated (n = 489) and relapsed/refractory patients with DLBCL (n = 117) and found that the co-occurrence of KMT2D and TP53 mutations was more prevalent in relapsed/refractory DLBCL (Figure 4D).44 Remarkably, among combinations of driver mutations, the frequency of the co-occurrence of KMT2D and TP53 mutations was by far the highest, accounting for 25.6% of relapsed/refractory cases (Figure 4E). These results suggest that co-occurring KMT2D and TP53 mutations were the most frequent event in DLBCL and confer negative prognostic impact.
Kmt2d and Trp53 deficiencies cooperate to cause B-cell lymphomas characterized by Yap1 overexpression
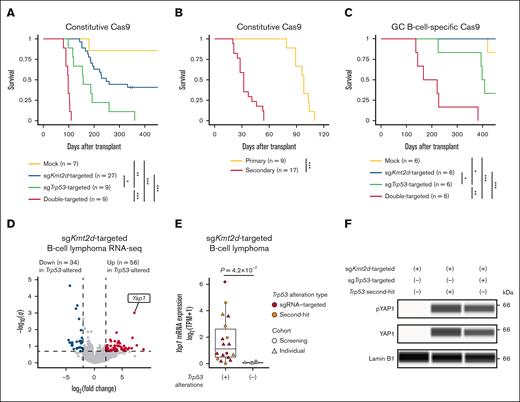
To substantiate the cooperative role of Kmt2d and Trp53 deficiencies, we transduced individual sgKmt2d or sgTrp53, or both, into HSPCs with constitutive Cas9 expression and transplanted them into lethally irradiated wild-type mice (supplemental Figure 9A; supplemental Table 7). Most recipient mice transplanted with single sgKmt2d- and sgTrp53-targeted HSPCs died of B-cell lymphoma and T-ALL/LBL, respectively (Figure 5A; supplemental Figure 9B). More importantly, all recipient mice transplanted with double sgKmt2d/sgTrp53-targeted HSPCs developed B-cell lymphoma, resulting in earlier death, with a median survival of 98 days (Figure 5A; supplemental Figure 9C). To confirm their lymphomagenic potential, we transplanted double sgKmt2d/sgTrp53-targeted tumor cells into sublethally irradiated recipients, all of which developed B-cell lymphoma and died within 58 days (Figure 5B). As KMT2D mutations are thought to arise in GC B cells,15,16 we replicated the experiment using HSPCs from Aicda-Cre; LSL-Cas9 mice with GC B-cell–specific Cas9 expression (supplemental Table 7). Although only few recipient mice transplanted with single sgKmt2d- and sgTrp53-targeted HSPCs developed lymphomas, all recipient mice transplanted with double sgKmt2d/sgTrp53-targeted HSPCs died of B-cell lymphoma with more extensive LAD, with a median survival of 194 days (Figure 5C; supplemental Figure 9D). WES analysis confirmed frequent occurrence of similar second-hit alterations in these tumors (Figure 5C; supplemental Figure 9E; supplemental Table 8). These findings suggest that combined inactivation of Kmt2d and Trp53 efficiently induces B-cell lymphomas recapitulating human diseases.
Kmt2d and Trp53 deficiencies coordinately induce B-cell lymphoma with Yap1 overexpression. (A) Survival curves of recipient mice transplanted with mock (n = 7), sgKmt2d (n = 27), sgTrp53 (n = 9), and double-targeted (n = 9) HSPCs with constitutive Cas9 expression. Log-rank test. (B) Survival curves of primary (n = 9) and secondary (n = 17) recipient mice transplanted with double sgKmt2d/sgTrp53-targeted HSPCs with constitutive Cas9 expression. Log-rank test. (C) Survival curves of recipient mice transplanted with mock (n = 6), sgKmt2d (n = 6), sgTrp53 (n = 6), and double-targeted (n = 6) HSPCs with GC B-cell–specific Cas9 expression. Log-rank test. (D) Volcano plot showing differentially expressed genes between single sgKmt2d-targeted (n = 7) and double sgKmt2d-targeted/Trp53-altered (n = 17) B-cell lymphoma. Genes with q value of <0.2 and |log2(fold change)| of >2 are considered significant and colored red (gained) or blue (lost). (E) Yap1 mRNA expression measured by RNA-seq in samples with (n = 17) or without (n = 7) Trp53 alterations in sgKmt2d-targeted B-cell lymphoma. Brunner-Munzel test. (F) YAP1 and pYAP1 protein expression measured by capillary electrophoresis-based immunoassay in representative samples with or without Trp53 alterations in sgKmt2d-targeted B-cell lymphoma. Lamin B1 is used as a loading control. Representative of 3 independent experiments. ∗P < .05; ∗∗P < .005; ∗∗∗P < .0005. mRNA, messenger RNA; pYAP1, phosphorylated YAP1; TPM, transcripts per million.
Kmt2d and Trp53 deficiencies coordinately induce B-cell lymphoma with Yap1 overexpression. (A) Survival curves of recipient mice transplanted with mock (n = 7), sgKmt2d (n = 27), sgTrp53 (n = 9), and double-targeted (n = 9) HSPCs with constitutive Cas9 expression. Log-rank test. (B) Survival curves of primary (n = 9) and secondary (n = 17) recipient mice transplanted with double sgKmt2d/sgTrp53-targeted HSPCs with constitutive Cas9 expression. Log-rank test. (C) Survival curves of recipient mice transplanted with mock (n = 6), sgKmt2d (n = 6), sgTrp53 (n = 6), and double-targeted (n = 6) HSPCs with GC B-cell–specific Cas9 expression. Log-rank test. (D) Volcano plot showing differentially expressed genes between single sgKmt2d-targeted (n = 7) and double sgKmt2d-targeted/Trp53-altered (n = 17) B-cell lymphoma. Genes with q value of <0.2 and |log2(fold change)| of >2 are considered significant and colored red (gained) or blue (lost). (E) Yap1 mRNA expression measured by RNA-seq in samples with (n = 17) or without (n = 7) Trp53 alterations in sgKmt2d-targeted B-cell lymphoma. Brunner-Munzel test. (F) YAP1 and pYAP1 protein expression measured by capillary electrophoresis-based immunoassay in representative samples with or without Trp53 alterations in sgKmt2d-targeted B-cell lymphoma. Lamin B1 is used as a loading control. Representative of 3 independent experiments. ∗P < .05; ∗∗P < .005; ∗∗∗P < .0005. mRNA, messenger RNA; pYAP1, phosphorylated YAP1; TPM, transcripts per million.
To gain insights into how Kmt2d and Trp53 deficiencies cooperatively induce B-cell lymphoma, we compared gene expression profiles between single sgKmt2d-targeted (n = 7) and double sgKmt2d/sgTrp53-targeted or Trp53-mutated (Trp53-altered) (n = 17) B-cell lymphoma samples from the CRISPR screening and the individual knockout experiments using RNA-seq. Differentially expressed gene analysis identified 56 upregulated and 34 downregulated genes in double sgKmt2d-targeted/Trp53-altered targeted B-cell lymphoma (Figure 5D; supplemental Table 9). Among them, the most highly expressed in double sgKmt2d/Trp53-altered B-cell lymphoma was Yap1, which is a transcriptional regulator downstream of the Hippo signaling pathway and whose upregulation promotes tumor progression in solid cancers.45,46 In sgKmt2d-targeted B-cell lymphoma, almost no Yap1 expression was observed in cases without Trp53 alterations, whereas Yap1 expression was strongly elevated in cases harboring Trp53 alterations, irrespective of whether they were sgRNA-targeted disruption or second-hit mutations (Figure 5E). These results were confirmed by increased yes-associated protein 1 (YAP1) expression in sgKmt2d-targeted/Trp53-altered B-cell lymphoma by capillary electrophoresis-based immunoassay (Figure 5F). Therefore, these results suggest that Yap1 overexpression is characteristic of Kmt2d- and Trp53-altered B-cell lymphoma.
Altered chromatin accessibility by co-occurring Kmt2d and Trp53 deficiencies in murine B-cell lymphoma
To clarify how the cooperation of Kmt2d and Trp53 deficiencies alters epigenetic status, we assessed chromatin accessibility by performing assay for transposase-accessible chromatin with sequencing for normal splenic B cells from wild-type mice (n = 6) as well as tumor cells from single sgKmt2d-targeted (n = 7), single Trp53-altered (n = 1), and sgKmt2d-targeted/Trp53-altered (n = 14) B-cell lymphoma and those without Kmt2d and Trp53 alterations (n = 7) from the CRISPR screening and the individual knockout experiments. Principal component analysis of assay for transposase-accessible chromatin with sequencing data clearly separated sgKmt2d-targeted B-cell lymphoma (irrespective of Trp53 alteration status) from normal B cells and B-cell lymphoma without Kmt2d and Trp53 alterations (Figure 6A). Differentially accessible region analysis identified 552 and 335 regions that gained and lost accessibility, respectively, in double sgKmt2d-targeted/Trp53-altered compared with single sgKmt2d-targeted B-cell lymphoma (Figure 6B; supplemental Table 10). Unsupervised clustering of differentially accessible regions revealed clear segregation of tumors with and without Trp53 alterations in sgKmt2d-targeted B-cell lymphoma (Figure 6C). Double sgKmt2d-targeted/Trp53-altered B-cell lymphoma showed progressive gain from single sgKmt2d-targeted B-cell lymphoma relative to normal B cells (Figure 6D). Importantly, gene expressions linked to gain or loss in chromatin accessibility in double sgKmt2d-targeted/Trp53-altered B-cell lymphoma were significantly upregulated or downregulated, respectively (Figure 6E). Proximal elements (−1000 base pairs to +100 base pairs of transcriptional start site), mainly consisting of promoters, were enriched in gained regions, but regions with greater gain of accessibility showed progressively increased representation of distal elements, including intergenic and intronic regions (supplemental Figure 10A-B). Motif enrichment analysis of distal regions revealed that 3 and 6 motifs were enriched in gained and lost regions, respectively (Figure 6F). Enriched transcription factors in lost regions included ETS1 and EBF1, critical regulators that are involved in B-cell differentiation and are recurrently mutated in DLBCL.6,7 Most importantly, enrichment of binding motif of transcriptional enhanced associate domain 1 (TEAD1), belonging to TEAD family of transcription factors, which interact with YAP1, was detected in gained regions (Figure 6F). Gene set enrichment analysis of expression data demonstrated that genes nearest to distal regions that gained accessibility and contained the TEAD1 motif were significantly upregulated in double sgKmt2d-targeted/Trp53-altered B-cell lymphoma (Figure 6G; supplemental Table 10). These observations suggest that Kmt2d- and Trp53-altered B-cell lymphoma shows a distinct chromatin accessibility status with TEAD1 motif enrichment.
Epigenetic changes in Kmt2d- and Trp53-altered B-cell lymphoma. (A) Principal component analysis plot of ATAC-seq data from normal splenic B cells from wild-type mice (n = 6) as well as tumor cells from single sgKmt2d-targeted (n = 7), single Trp53-altered (n = 1), and double sgKmt2d-targeted/Trp53-altered (n = 14) B-cell lymphomas and those without Kmt2d and Trp53 alterations (n = 7). (B) Volcano plot showing DARs between single sgKmt2d-targeted (n = 7) and double sgKmt2d-targeted/Trp53-altered (n = 14) B-cell lymphoma. Merged ATAC-seq peaks with q value of <0.2 and |fold change| of >1.5 are considered significant and colored red (gained) or blue (lost). (C) Heat map showing DARs between single sgKmt2d-targeted and double sgKmt2d/Trp53-altered B-cell lymphoma. Unsupervised hierarchical clustering was performed with Pearson correlation and Ward.D2 linkage algorithm. (D) Mean normalized ATAC-seq signal intensity for regions that gained accessibility in double sgKmt2d-targeted/Trp53-altered B-cell lymphoma compared with single sgKmt2d-targeted B-cell lymphoma. (E) Cumulative frequency of expression for genes nearest to regions that gained or lost accessibility or remained stable in double sgKmt2d-targeted/Trp53-altered B-cell lymphoma compared with single sgKmt2d-targeted B-cell lymphoma. Kolmogorov-Smirnov test. (F) Motif enrichment analysis for distal regions (−1000 base pairs [bp] to +100 bp of TSS) that gained or lost accessibility in double sgKmt2d-targeted/Trp53-altered B-cell lymphoma compared with single sgKmt2d-targeted B-cell lymphoma. (G) Gene set enrichment analysis of expression data comparing single sgKmt2d-targeted and double sgKmt2d-targeted/Trp53-altered B-cell lymphoma using genes nearest to distal regions that gained accessibility and contained the TEAD1 motif. ATAC-seq, assay for transposase-accessible chromatin with sequencing; DAR, differentially accessible region; EBF, early B cell factor; ES, enrichment score; Ets-1, ETS proto-oncogene 1; FDR, false discovery rate; Hoxa9, homeobox A9; mRNA, messenger RNA; NFIA, nuclear factor I A; Oct6, organic cation/carnitine transporter 6; PC1/2, principal component 1/2; Pft1a, pancreas associated transcription factor 1a; PGR, progesterone receptor; RUNX, runt-related transcription factor; TSS, transcriptional start site.
Epigenetic changes in Kmt2d- and Trp53-altered B-cell lymphoma. (A) Principal component analysis plot of ATAC-seq data from normal splenic B cells from wild-type mice (n = 6) as well as tumor cells from single sgKmt2d-targeted (n = 7), single Trp53-altered (n = 1), and double sgKmt2d-targeted/Trp53-altered (n = 14) B-cell lymphomas and those without Kmt2d and Trp53 alterations (n = 7). (B) Volcano plot showing DARs between single sgKmt2d-targeted (n = 7) and double sgKmt2d-targeted/Trp53-altered (n = 14) B-cell lymphoma. Merged ATAC-seq peaks with q value of <0.2 and |fold change| of >1.5 are considered significant and colored red (gained) or blue (lost). (C) Heat map showing DARs between single sgKmt2d-targeted and double sgKmt2d/Trp53-altered B-cell lymphoma. Unsupervised hierarchical clustering was performed with Pearson correlation and Ward.D2 linkage algorithm. (D) Mean normalized ATAC-seq signal intensity for regions that gained accessibility in double sgKmt2d-targeted/Trp53-altered B-cell lymphoma compared with single sgKmt2d-targeted B-cell lymphoma. (E) Cumulative frequency of expression for genes nearest to regions that gained or lost accessibility or remained stable in double sgKmt2d-targeted/Trp53-altered B-cell lymphoma compared with single sgKmt2d-targeted B-cell lymphoma. Kolmogorov-Smirnov test. (F) Motif enrichment analysis for distal regions (−1000 base pairs [bp] to +100 bp of TSS) that gained or lost accessibility in double sgKmt2d-targeted/Trp53-altered B-cell lymphoma compared with single sgKmt2d-targeted B-cell lymphoma. (G) Gene set enrichment analysis of expression data comparing single sgKmt2d-targeted and double sgKmt2d-targeted/Trp53-altered B-cell lymphoma using genes nearest to distal regions that gained accessibility and contained the TEAD1 motif. ATAC-seq, assay for transposase-accessible chromatin with sequencing; DAR, differentially accessible region; EBF, early B cell factor; ES, enrichment score; Ets-1, ETS proto-oncogene 1; FDR, false discovery rate; Hoxa9, homeobox A9; mRNA, messenger RNA; NFIA, nuclear factor I A; Oct6, organic cation/carnitine transporter 6; PC1/2, principal component 1/2; Pft1a, pancreas associated transcription factor 1a; PGR, progesterone receptor; RUNX, runt-related transcription factor; TSS, transcriptional start site.
Targeting YAP1 is effective against KMT2D- and TP53-altered B-cell lymphoma
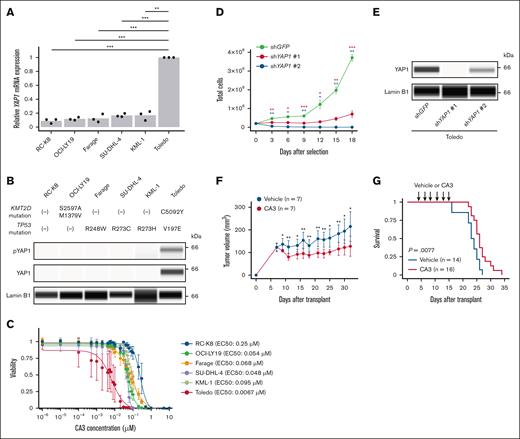
The aforementioned findings led us to explore the therapeutic potential of YAP1 inhibition against KMT2D/TP53-altered B-cell lymphoma. In human DLBCL cell lines, Toledo cells (with both KMT2D and TP53 mutations) had strong YAP1 messenger RNA and protein expression, whereas RC-K8 (without KMT2D and TP53 mutations); OCI-LY19 (with KMT2D mutations); as well as Farage, SU-DHL-4, and KML-1 (with TP53 mutations) showed weak or no YAP1 expression (Figure 7A-B). We investigated the effect of YAP1 inhibition with CA3, a potent YAP1/TEAD inhibitor, against these cell lines in vitro and found that CA3 efficiently inhibited the viability of Toledo cells compared with all other cell lines (Figure 7C). Consistent with these findings, we also demonstrated that short hairpin RNA–mediated YAP1 knockdown significantly suppressed the proliferation of Toledo cells (Figure 7D-E). Then, we transplanted Toledo cells into BALB/c-nu/nu mice and examined their response to CA3. We found that CA3 efficiently suppressed tumor growth in the xenotransplantation model (Figure 7F). Finally, we administered CA3 to recipient mice of double sgKmt2d/sgTrp53-targeted B-cell lymphoma cells and found that CA3 significantly prolonged their survival (Figure 7G). Taken together, YAP1 overexpression can be a potential therapeutic target in KMT2D- and TP53-altered B-cell lymphoma.
Yap1 overexpression and its targeting in Kmt2d- and Trp53-mutated B-cell lymphoma. (A) YAP1 mRNA expression measured by real-time quantitative polymerase chain reaction in 6 human DLBCL cell lines (n = 3 for each) with and without KMT2D and/or TP53 mutations. (B) YAP1 and pYAP1 protein expression measured by capillary electrophoresis-based immunoassay in 6 human DLBCL cell lines. Lamin B1 is used as a loading control. Representative of 3 independent experiments. (C) Effect of CA3 treatment on viability of 6 human DLBCL cell lines (n = 3 for each) measured by CellTiter-Glo luminescent cell viability assay. (D) Cell proliferation of Toledo cells expressing short hairpin RNA (shRNA) targeting GFP (control) or YAP1 after puromycin selection. (E) YAP1 protein expression measured by capillary electrophoresis-based immunoassay in Toledo cells expressing shRNA targeting GFP or YAP1. Lamin B1 is used as a loading control. Representative of 2 independent experiments. (F) Tumor volume in Toledo xenograft mice administered with 4 mg/kg of CA3 (n = 7) or vehicle (n = 7) 3 times a week for 12 doses. (G) Survival curves of mice transplanted with 2.5 × 103 murine double sgKmt2d-targeted/Trp53-altered B-cell lymphoma cells and administered with 4 mg/kg of CA3 (n = 16) or vehicle (n = 14) 3 times a week for 6 doses. Log-rank test. In panels C-D,F, data are represented as mean ± standard deviation. In panels A,D,F, Welch t test was performed. ∗P < .05; ∗∗P < .005; ∗∗∗P < .0005.
Yap1 overexpression and its targeting in Kmt2d- and Trp53-mutated B-cell lymphoma. (A) YAP1 mRNA expression measured by real-time quantitative polymerase chain reaction in 6 human DLBCL cell lines (n = 3 for each) with and without KMT2D and/or TP53 mutations. (B) YAP1 and pYAP1 protein expression measured by capillary electrophoresis-based immunoassay in 6 human DLBCL cell lines. Lamin B1 is used as a loading control. Representative of 3 independent experiments. (C) Effect of CA3 treatment on viability of 6 human DLBCL cell lines (n = 3 for each) measured by CellTiter-Glo luminescent cell viability assay. (D) Cell proliferation of Toledo cells expressing short hairpin RNA (shRNA) targeting GFP (control) or YAP1 after puromycin selection. (E) YAP1 protein expression measured by capillary electrophoresis-based immunoassay in Toledo cells expressing shRNA targeting GFP or YAP1. Lamin B1 is used as a loading control. Representative of 2 independent experiments. (F) Tumor volume in Toledo xenograft mice administered with 4 mg/kg of CA3 (n = 7) or vehicle (n = 7) 3 times a week for 12 doses. (G) Survival curves of mice transplanted with 2.5 × 103 murine double sgKmt2d-targeted/Trp53-altered B-cell lymphoma cells and administered with 4 mg/kg of CA3 (n = 16) or vehicle (n = 14) 3 times a week for 6 doses. Log-rank test. In panels C-D,F, data are represented as mean ± standard deviation. In panels A,D,F, Welch t test was performed. ∗P < .05; ∗∗P < .005; ∗∗∗P < .0005.
Discussion
Through CRISPR loss-of-function screening, we delineated oncogenic potential of recurrently mutated genes in DLBCL and their association with lineage, demonstrating strong oncogenic capacity of Kmt2d, Pax5, and Trp53 in B-cell lymphoma. We have also elucidated cooperability between sgRNA-targeted genes and second-hit alterations, discovering the cooperation of Kmt2d and Trp53 deficiencies to induce B-cell lymphomas. KMT2D, encoding the lysine-specific histone methyltransferase, is 1one of the most frequently mutated genes in DLBCL and enriched in the EZB subtype.6,7,13Kmt2d disruption is reported to enhance GC B-cell proliferation and increase the occurrence of B-cell lymphomas in mice overexpressing Bcl2.15,16 In contrast, TP53, a well-known tumor suppressor, is also frequently mutated in DLBCL and characterizes the A53 subtype.6,13 Conditional inactivation of Trp53 in mature B cells promotes generation of B-cell lymphomas after long latency.14 Although KMT2D and TP53 mutations characterize different genetic subtypes,6,13 we reveal that these 2 mutations are the most frequent co-occurring combination and associated with worse prognosis in human DLBCL. Remarkably, the frequency of KMT2D and TP53 mutations is increased in relapsed/refractory DLBCL, accounting for approximately one-fourth of such cases. In addition, combined Kmt2d and Trp53 knockout efficiently causes B-cell lymphomas resembling human diseases in mice. These observations suggest that KMT2D and TP53 mutations confer the most aggressive phenotype, underscoring the necessity for novel treatment strategies against tumors with this combination.
Notably, our transcriptomic and epigenomic sequencing have revealed Yap1 overexpression and TEAD1 motif enrichment in open chromatin regions in double sgKmt2d-targeted/Trp53-altered B-cell lymphoma. YAP1, a key oncogenic driver in the Hippo pathway, is frequently activated by amplifications and gene fusions and its overexpression is associated with poor prognosis in various solid cancers.45,46 A considerable body of literature demonstrates functional relationships between the YAP1/Hippo and p53 pathways in oncogenesis.47,48 Particularly, TP53 status serves as the molecular switch for YAP1 between tumor suppressive and oncogenic functions: the YAP1/Hippo pathway cooperates with wild-type p53 to induce cell cycle arrest and apoptosis, contributing to the maintenance of genome integrity, whereas YAP1/Hippo pathway drives tumorigenesis in cells with no or aberrant p53 activity.49,50 In addition, Trp53 loss is reported to induce Yap1 transcriptional upregulation through regulating Ptpn14 in pancreatic cancer.51 Therefore, the YAP1/Hippo pathway activation and Trp53 loss are closely coordinated through multiple components of their pathways and involved in the development and progression of various malignancies, including lymphomas. Given compounds targeting YAP1/TEAD interaction have shown strong efficacy against murine solid cancers and are currently under clinical development,45,46,52 our findings suggest that YAP1 inhibition can be a promising therapeutic option for DLBCL harboring KMT2D and TP53 mutations.
In vivo CRISPR screening is a powerful approach to simultaneously evaluate the roles of a number of loss-of-function alterations.31,32 In addition to assessing in vivo tumorigenic potential of recurrently mutated genes in DLBCL, we have identified a variety of second-hit alterations, including mutations, amplifications, and gene fusions, which showed significant associations with disease phenotype and sgRNA-targeted genes. Besides enrichment of Trp53 alterations in sgKmt2d-targeted B-cell lymphomas, frequent alterations involving FLT3, JAK/STAT, and RAS pathways were observed in sgPax5-targeted B-cell malignancies, consistent with human B-ALL/LBL. In addition, C-terminal truncating mutations of Notch1 frequently occurred in sgSocs1-targeted T-ALL/LBL, which is consistent with human peripheral T-cell lymphoma, not otherwise specified, showing the co-occurrence of SOCS1 and NOTCH1 mutations.36 These observations demonstrate that second-hit alterations in mouse models successfully recapitulate the mutation spectrum seen in human cancers, although altered genes within the pathways are to some extent different between species. Thus, a combined strategy of in vivo CRISPR screening and integrative genomic profiling enables high-throughput exploration of cooperative roles of driver alterations. Given hundreds of recurrently mutated genes have been reported in DLBCL,5-10 further studies investigating a larger number of candidate drivers would be warranted.
In conclusion, our in vivo CRISPR loss-of-function screening has enabled high-throughput evaluation of the tumorigenic potential of many genetic abnormalities and their functional relationships, demonstrating the cooperation between KMT2D and TP53 disruptions, in DLBCL. Such an approach accelerates modeling human cancers, understanding of genotype-phenotype association, and discovery of potential therapeutic targets.
Acknowledgments
The authors thank Akira Nishiyama and Tomohiko Tamura at Yokohama City University for their valuable advice on assay for transposase-accessible chromatin with sequencing experiment; Hitoshi Ichikawa, Sachiyo Mitani, and Fundamental Innovative Oncology Core, National Cancer Center Research Institute, for sequencing analyses; and Fumie Ueki, Yoko Hokama, and Yoshiko Ito for their technical assistance. The supercomputing resources were provided by the Human Genome Center, the Institute of Medical Science, The University of Tokyo. The images of mice in Figure 1A are from TogoTV (2016 Database Center for Life Science TogoTV).
This work is supported by Japan Society for the Promotion of Science KAKENHI grants JP21H05051, JP18H04035, and JP18K16079, Japan Science and Technology Agency Moonshot R&D Program (JPMJMS2022), Takeda Science Foundation, the Uehara Memorial Foundation, and National Cancer Center Research and Development Funds (2020-A-5). The Genomic Variation in Diffuse Large B Cell Lymphomas study (phs001444.v2.p1) was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health (NIH), Department of Health and Human Services. The data sets have been accessed through the NIH database for Genotypes and Phenotypes. A full list of acknowledgments can be found in the supplemental note (available at 10.1056/NEJMoa1801445).
Authorship
Contribution: J.K. and K.K. designed the study; K. Yamaguchi, K. Yoshifuji, Y.K., and J.K. performed experiments; K. Mizuno, K. Yamaguchi, Y.M., Y. Saito, Y.K., and K.K. performed sequencing data analyses; K.C., A.O., and Y. Shiraishi developed sequence data processing pipelines; S.S. and K. Murakami assisted experiments; M.T., M.Y., Y.I., K.D., and R.M. assisted data analyses; K.N. and K.O. performed pathological analyses; K. Yamaguchi, K. Mizuno, J.K., Y.M., Y.K., and K.K. generated figures and tables, and wrote the manuscript; K.K. led the entire project; and all authors participated in discussions and interpretation of the data and results.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Keisuke Kataoka, Division of Molecular Oncology, National Cancer Center Research Institute, 5-1-1 Tsukiji, Chuo-ku, Tokyo, 104-0045, Japan; email: kkataoka-tky@umin.ac.jp.
References
Author notes
K. Yamaguchi, J.K., and K. Mizuno contributed equally to this study.
Mouse whole-exome sequencing, RNA-sequencing, and assay for transposase-accessible chromatin with sequencing data have been deposited with links to BioProject accession number PRJDB17514 in the DNA Data Bank of Japan BioProject database.
The full-text version of this article contains a data supplement.


![Epigenetic changes in Kmt2d- and Trp53-altered B-cell lymphoma. (A) Principal component analysis plot of ATAC-seq data from normal splenic B cells from wild-type mice (n = 6) as well as tumor cells from single sgKmt2d-targeted (n = 7), single Trp53-altered (n = 1), and double sgKmt2d-targeted/Trp53-altered (n = 14) B-cell lymphomas and those without Kmt2d and Trp53 alterations (n = 7). (B) Volcano plot showing DARs between single sgKmt2d-targeted (n = 7) and double sgKmt2d-targeted/Trp53-altered (n = 14) B-cell lymphoma. Merged ATAC-seq peaks with q value of <0.2 and |fold change| of >1.5 are considered significant and colored red (gained) or blue (lost). (C) Heat map showing DARs between single sgKmt2d-targeted and double sgKmt2d/Trp53-altered B-cell lymphoma. Unsupervised hierarchical clustering was performed with Pearson correlation and Ward.D2 linkage algorithm. (D) Mean normalized ATAC-seq signal intensity for regions that gained accessibility in double sgKmt2d-targeted/Trp53-altered B-cell lymphoma compared with single sgKmt2d-targeted B-cell lymphoma. (E) Cumulative frequency of expression for genes nearest to regions that gained or lost accessibility or remained stable in double sgKmt2d-targeted/Trp53-altered B-cell lymphoma compared with single sgKmt2d-targeted B-cell lymphoma. Kolmogorov-Smirnov test. (F) Motif enrichment analysis for distal regions (−1000 base pairs [bp] to +100 bp of TSS) that gained or lost accessibility in double sgKmt2d-targeted/Trp53-altered B-cell lymphoma compared with single sgKmt2d-targeted B-cell lymphoma. (G) Gene set enrichment analysis of expression data comparing single sgKmt2d-targeted and double sgKmt2d-targeted/Trp53-altered B-cell lymphoma using genes nearest to distal regions that gained accessibility and contained the TEAD1 motif. ATAC-seq, assay for transposase-accessible chromatin with sequencing; DAR, differentially accessible region; EBF, early B cell factor; ES, enrichment score; Ets-1, ETS proto-oncogene 1; FDR, false discovery rate; Hoxa9, homeobox A9; mRNA, messenger RNA; NFIA, nuclear factor I A; Oct6, organic cation/carnitine transporter 6; PC1/2, principal component 1/2; Pft1a, pancreas associated transcription factor 1a; PGR, progesterone receptor; RUNX, runt-related transcription factor; TSS, transcriptional start site.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/19/10.1182_bloodadvances.2024015519/2/m_blooda_adv-2024-015519-gr6.jpeg?Expires=1769314251&Signature=aMpMG3ADAI12R6byS0YPYehxc7DuX~ZOTEmPH38dxU2lun4YJphmaGCUmV6qg10L5yrjcVeOndRQ8RTBp-buz9Z4Di2UuYFkq~U9md4nDPsJwo5lKxvgBVdTZC~2LGtcR4r8w68LHxttOe3idsEzXGSgO-bV7CRh72RVP1smcd-eJIR1v~ooR7j7wqlSkifewyroqTEtrAbztm5Aor-8El2EHj4oKwy1mkxbI2f5J4GH8CJJN7kug5V9oIDG0xC1m03jEVu9Zcvurbf5-~xOIIxhGhKpPM5s9UfmeJnXQ75npTQ4h62JiX3BwbhROBnEBsDMTuN2WFmSCrVcEjm8Vw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)