Key Points
Pirfenidone is safe and tolerable in persons with BOS.
Pirfenidone may improve lung function in patients with BOS as measured by percent predicted forced expiratory volume in 1 second.
Visual Abstract
Bronchiolitis obliterans syndrome (BOS) is a severe form of chronic graft-versus-host disease (cGVHD) following allogeneic hematopoietic cell transplant (HCT), with a 5-year survival rate of 40%. Currently, there is no curative therapy for BOS. Preclinical data suggest that pirfenidone, an antifibrotic drug, may benefit small airway fibrosis in HCT-associated BOS. A single-arm, open-label, 56-week phase 1 trial with 56-month extension evaluated pirfenidone’s tolerability, safety, and efficacy in patients with BOS. Efficacy was measured using pulmonary function tests (PFTs), quantitative computed tomography (qCT) scans, patient-reported outcomes (PROs), cGVHD indices, and laboratory tests. Lung function trajectory was assessed by change in regression slopes before and during treatment. Baseline qCT metrics, including percentage normal lung, air trapping, volume change (Jacobian), and heterogeneity of volume change (Jacobian variance), were analyzed by participant response. Of 30 participants, 25 completed the 56-week trial, and 10 continued into the extension. Overall, 63% tolerated the recommended dose without safety concerns. There was significant improvement in the percent predicted forced expiratory volume in 1 second (P = .00267) when analyzing all participants, and improvement in individual PFT trend for 41.3% of participants. qCT analysis by lobe showed healthier lungs in the upper lobes of responders. Significant improvements were noted in liver function tests, PROs related to physical functioning and shortness of breath, and cGVHD skin indices. These findings indicate that pirfenidone is safe and tolerable in patients with BOS after HCT and may improve lung function and symptoms. Further trials are warranted to evaluate the efficacy of pirfenidone as a treatment for BOS after HCT. This trial was registered at www.ClinicalTrials.gov as #NCT03315741.
Introduction
Bronchiolitis obliterans syndrome (BOS) is a highly morbid, progressive form of chronic graft-versus-host disease (cGVHD) that occurs after allogeneic hematopoietic cell transplant (HCT), affecting ∼5% to 15% of patients,1 and with a 2-year and 5-year survival rate of 60% and 40%, respectively.2,3 BOS is characterized by peribronchiolar inflammation, extracellular matrix deposition, and fibrosis resulting in bronchial narrowing.1 Subsequent irreversible airway obstruction leads to dyspnea, poor quality of life, and often death.4,5
Currently, the primary treatment for BOS involves inhaled corticosteroids, azithromycin, and montelukast.6,7 This regimen has not been shown to alter disease progression, and carries a conditional recommendation with very low certainty of evidence by European Respiratory Society and European Society for Blood and Marrow Transplantation guidelines.5,8 Systemic steroids are also used, with clinical responses estimated to occur in only 50% of patients, and sustained high-dose usage has diminishing returns in improving lung function while increasing risk of infection and other complications.4 Potent immunosuppressive agents provide viable alternatives, but their use is complicated by high infection rates, which can increase morbidity and nonrelapse mortality.4 These treatment limitations highlight the imperative to identify novel treatments for BOS.
Pirfenidone is an antifibrotic drug currently approved by the US Food and Drug Administration for the treatment of idiopathic pulmonary fibrosis (IPF).9 Its primary mechanism of action involves attenuation of transforming growth factor-β–stimulated collagen synthesis and reduction of fibroblast proliferation.10 Prior case reports describe the efficacy of pirfenidone in patients with lung transplant complicated by chronic lung allograft dysfunction (CLAD),11 and preclinical studies suggest a potential role for pirfenidone in BOS following HCT.12 Together, these observations prompted the conduct of a prospective study to evaluate pirfenidone as therapy for BOS. We have previously published an interim analysis of 22 patients from a single-arm, single-center, open-labeled, 12-month, phase 1 trial of pirfenidone in persons with HCT-BOS, showing that pirfenidone was tolerated and associated with pulmonary function test (PFT) improvement for 38% of participants over a 12-month period.13 Here we present results from all 30 participants and a 56-month extension trial to evaluate the long-term tolerability and efficacy of pirfenidone for BOS. The primary outcomes were safety and tolerability of pirfenidone at the recommended dose used for treatment of IPF. Secondary study objectives included an assessment of treatment efficacy by PFTs, quantitative computed tomography (qCT) chest scan, laboratory tests, validated patient-reported outcome (PRO) measures, and cGVHD indices.
Methods
Study design and oversight
Enrollment was conducted at Stanford University Medical Center from March 2018 to February 2021. Titration of pirfenidone was conducted in 2 phases: a 3-week drug titration period (801 mg/day in 3 divided doses in week 1, 1602 mg/day in week 2, and 2403 mg/day in week 3); followed by a 49-week active treatment period at a stable, tolerated dose.13 The extension trial allowed participants to continue pirfenidone up to a total study duration of 56 months, and ended in October 2022. PFTs, PROs, and cGVHD indices were collected for all participants during the extension period. qCT scans of the chest were done at trial enrollment. Data were collected and analyzed in compliance with the Health Insurance Portability and Accountability Act. The study protocol was approved by Stanford’s Institutional Review Board (IRB no. 43319) and Scientific Review Committee and monitored annually by the Data Safety Monitoring Committee of the Stanford Cancer Institute. All trial participants provided written informed consent. The study is registered in ClinicalTrials.gov (NCT03315741).
Study participants
Patients enrolled in the study were adults aged ≥18 years, and were seen in the Stanford Lung GVHD Clinic with a diagnosis of BOS based on 1 of the following: (1) National Institutes of Health (NIH) criteria,14 (2) a lung biopsy consistent with obliterative bronchiolitis, or (3) clinical criteria as defined as a progressive decline in forced expiratory volume in 1 second (FEV1) not otherwise explained and not yet meeting the NIH criteria for BOS.5 Notable exclusion criteria were uncontrolled infections, recent surgery, and life expectancy ≤6 months. Full inclusion and exclusion criteria are provided in the supplemental Data (supplemental Table 1).
Safety assessments and outcome measures
Safety assessments included monitoring of adverse events (AEs), clinical labs, vital signs, electrocardiograms, and physical examinations. Study participants logged AEs into patient diaries. AEs and severe AEs (SAEs) were graded according to the National Cancer Institute Common Terminology Criteria of Adverse Events version 4.0. All AEs and SAEs were reported to the IRB and Stanford’s Cancer Clinical Trials Office.
The primary outcome was drug tolerability, measured as participants able to maintain pirfenidone’s recommended dose for IPF (2403 mg/day)10 without requiring dose reduction longer than 21 days due to AEs or SAEs. The prespecified tolerability threshold to justify proceeding with a future randomized controlled trial was set at 25%, considering overlap in cGVHD symptoms with the effects of pirfenidone. Secondary outcomes included treatment-emergent AEs, PFT trajectory, qCT metrics, laboratory results, PROs, and cGVHD indices. PROs were measured using the Medical Outcomes Study Short Form-36 (SF-36)15 and the University of California San Diego Shortness of Breath Questionnaire (UCSD SOBQ).16 Indices for cGVHD were measured using the NIH cGVHD clinical score14 and the Lee cGVHD Symptom Scale (LSS).17 The primary and secondary outcomes were assessed during study visits at 1- to 3-month intervals, following standard of care (SOC) practices.
Trial procedures
Enrolled participants received pirfenidone with dosing documented and verified by pill counts in patient diaries. Study visits occurred at a minimum of every 3 months, during which we recorded forced vital capacity (FVC) and FEV1 (normalized as percent predicted per accepted National Health and Nutrition Survey reference equations),18 complete blood counts, metabolic panels (including renal function and electrolytes), and liver function tests (LFTs; aspartate transaminase [AST], alanine transaminase [ALT], and alkaline phosphatase [ALP]). These parameters were also collected from 24 months prior to study enrollment to perform longitudinal assessment, with positive response defined as a statistically significant difference in regression lines on drug compared with the pretrial period. Paired inspiratory/expiratory CT scans of the chest were performed at trial enrollment and analyzed with algorithmic postprocessing to generate quantitative output. Questionnaires for PROs (SF-36, UCSD SOBQ) and for cGHVD indices by the NIH-GVHD scale and the LSS were administered at baseline, 6 months, and 12 months, with UCSD SOBQ also collected at the end of the extension trial. For each PRO, a minimum clinically important difference (MCID) was defined as a change from baseline of ≥5 points.19
Immune modulation, infection, and nonpulmonary GVHD flare
Concomitant medications for BOS and cGVHD based on SOC were allowed. Immune modulatory medication usage was tracked throughout the 12-month trial and extension trial. The average daily prednisone dose was calculated for the month preceding each PFT, along with a binary label for ruxolitinib and belumosudil use in the same period. Cumulative exposure to prednisone was calculated. Infections and their pathogens were recorded weekly, with an infection noted if it occurred within 1 month of a PFT. Indolent infections, such as nontuberculous mycobacterium, were counted only at onset.
qCT chest scans
A total of 23 patients received paired inspiratory/expiratory CT scans at enrollment, and were analyzed by qCT for percentage normal lung, percentage air trapping, lung volume expansion (Jacobian), and intra-lung heterogeneity of volume expansion (Jacobian variance), which are previously validated metrics.20-22 qCT metrics were calculated as whole-lung means and segmented by lobes (right upper, right middle, right lower, left upper, and left lower).
Statistical analysis
Study data were collected and managed using a Research Electronic Data Capture electronic (REDCap) database and analyzed with R version 4.4.2. Categorical variables were analyzed using Fisher exact test for n 5 or a c2 test for n >5. Continuous variables were analyzed by 2-sided t tests with Welch’s correction, or 2-sample Wilcoxon rank-sum tests for nonparametric data. LFTs were analyzed by a 2-way analysis of variance to assess the effect of pirfenidone treatment. To assess change in percent predicted FEV1 (ppFEV) or percent predicted FVC (ppFVC), individual linear regression slopes were compared for 24 months prior to enrollment with the period when a participant was on drug.5 A 24-month pretrial period was chosen to include at least 2 PFTs per participant to establish a baseline trajectory, and reflect long-term follow-up. Individual PFT trajectories were modeled using best-fit lines with trend smoothing. Grouped linear regression lines and 95% confidence intervals were also calculated, with P values ≤.05 considered significant. Pearson correlation was used to assess the association of PFT with immune modulatory medications, infections, and cGVHD flares. Conditional inference trees (CITs) were applied to qCT metrics as covariates, and responder status as outcome. Additional details on statistical analysis are provided in supplemental Data.
Results
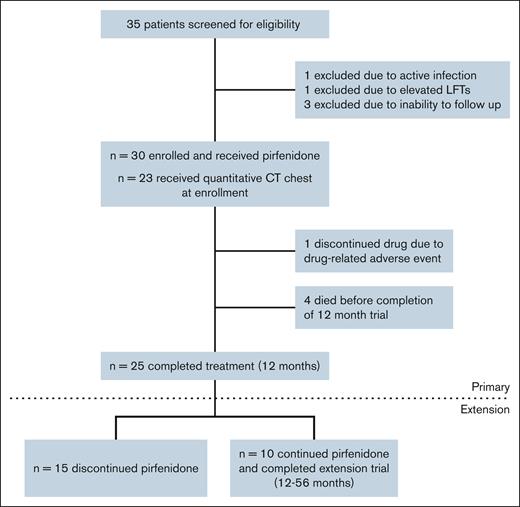
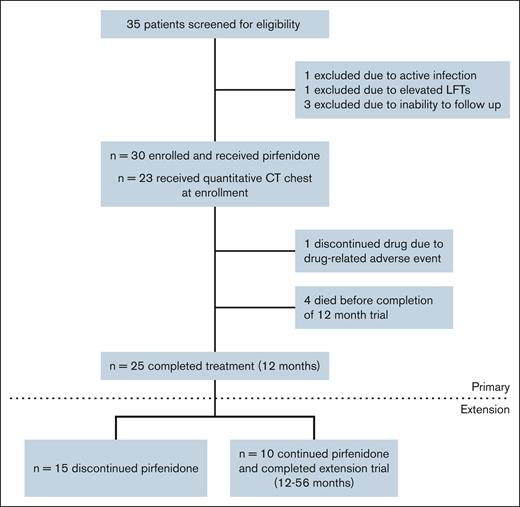
Figure 1 shows the study enrollment flowchart for the 56-week trial and the 56-month extension. Table 1 provides baseline and disease characteristics for the 30 enrolled participants. Of the participants, 15 (50%) were female, with a mean age of 54.4 years at enrollment. In the extension group (n = 10), 5 were female, with a mean age of 53.7 years. BOS was diagnosed in 27 patients using NIH consensus criteria (26 by PFT criteria and 1 by lung biopsy), and in 3 patients by clinical criteria. The median time from HCT to BOS diagnosis was 16.2 months (interquartile range [IQR], 9.1-28.1), and from BOS diagnosis to trial enrollment was 28.5 months (IQR, 13.9-56.8). Participants had moderate baseline pulmonary impairment, with mean ppFVC 72% and ppFEV1 52%. All patients were treated with inhaled corticosteroids and montelukast. At enrollment, prednisone was the most common systemic immunosuppressant for cGVHD and BOS, followed by tacrolimus, mycophenolate mofetil, and ibrutinib. Four participants died during the 12-month trial, but none died during the extension trial.
Tolerability and safety
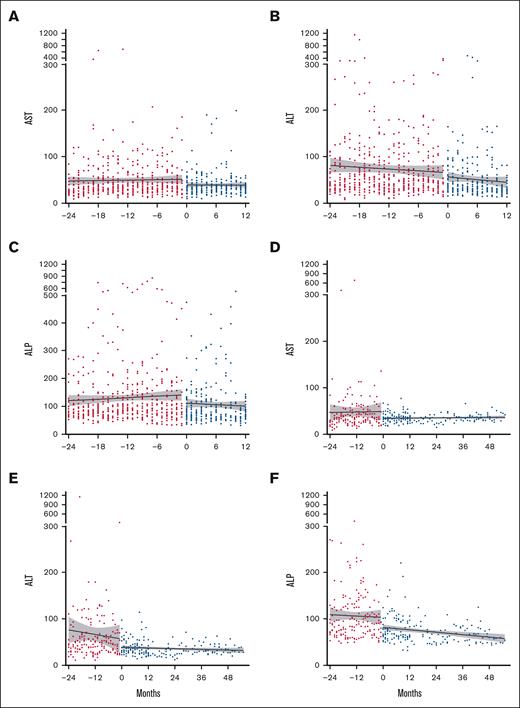
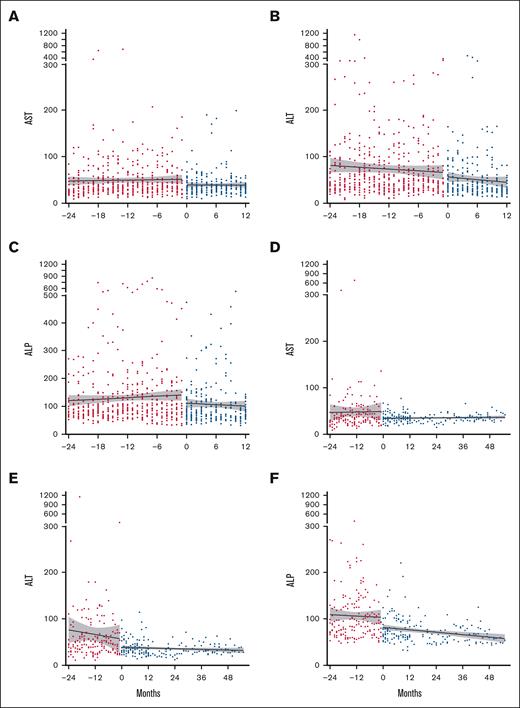
Most participants tolerated daily pirfenidone. Of the 30 individuals who started treatment, 19 (63.3.%) tolerated the recommended daily dose (2403 mg/day), 9 (30%) tolerated a lower dose, and 2 (6.7%) were drug intolerant (<1 capsule per day). Table 2 details AEs and SAEs throughout the trial. Fatigue (83.3%) was the most common AE and was typically self-limiting. Gastrointestinal (GI) AEs frequently occurred: nausea (60%), weight loss (36.7%), and gastroesophageal reflux disorder (36.7%). Twelve participants (40%) reported 23 total SAEs, 87% of which were infections, including 4 infection-associated deaths during the 12-month trial. Pirfenidone was discontinued in 1 case due to a photosensitive skin rash following a Sarcoptes scabiei infection; this participant had previously tolerated pirfenidone for several months. All 10 participants who continued during the extension survived and tolerated pirfenidone. No significant before and after treatment differences were observed in complete blood count, electrolytes, or renal function. Most participants had mild baseline elevations in AST, ALT, and ALP. Two participants developed elevated LFTs (≥3 upper limit of normal), which resolved with dosage modification. Overall, pirfenidone treatment was associated with a decrease in all liver enzymes (P < .0001; Figure 2).
Improvement in LFTs with pirfenidone treatment. LFTs: (A) AST, (B) ALT, and (C) ALP were compared between pretreatment levels (24 months prior to starting pirfenidone, shown in red circles) and levels during treatment (0-12 months, shown in blue circles) for all participants. Data for the 10 participants who continued in the extension trial: (D) AST, (E) ALT, and (F) ALP levels. Data are presented as best-fit lines with 95% confidence intervals in gray, analyzed by 2-way analysis of variance. All LFTs showed significant improvement with P < .0001. AST, aspartate transaminase.
Improvement in LFTs with pirfenidone treatment. LFTs: (A) AST, (B) ALT, and (C) ALP were compared between pretreatment levels (24 months prior to starting pirfenidone, shown in red circles) and levels during treatment (0-12 months, shown in blue circles) for all participants. Data for the 10 participants who continued in the extension trial: (D) AST, (E) ALT, and (F) ALP levels. Data are presented as best-fit lines with 95% confidence intervals in gray, analyzed by 2-way analysis of variance. All LFTs showed significant improvement with P < .0001. AST, aspartate transaminase.
PFTs
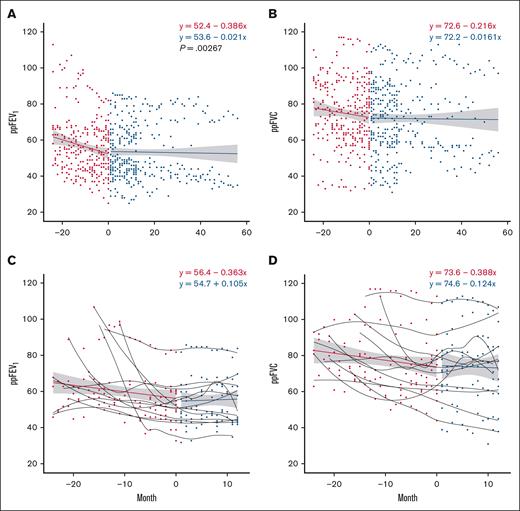
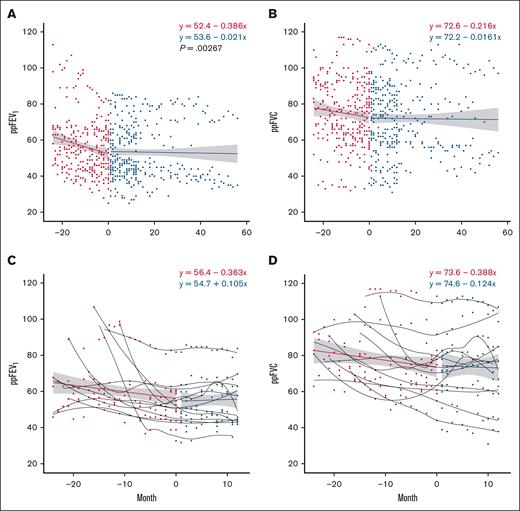
Spirometry during the trial was compared with spirometry obtained up to 24 months before starting pirfenidone, with a minimum of 3 and a maximum of 19 PFTs obtained during the trial period (median [IQR], 12 [5-19]). The 29 participants included in the analysis received a PFT a minimum of every 3 months while on study; the results are shown in Figure 3. Pirfenidone significantly increased ppFEV1 slope when comparing baseline and after enrollment spirometry; this effect persisted in all participants during the 12-month trial, and in the 10 individuals in the extension trial (Figure 3A; P = .003). The trend in ppFVC also suggested disease stabilization, with a less negative regression slope while on drug, but this was not statistically significant (Figure 3B; P = .170). Analysis of individual ppFEV1 and ppFVC slopes comparing pretrial with on-drug showed significant improvement in 12 participants (41.3%), who were considered “responders” to pirfenidone (supplemental Figure 1). Composite regression lines of responders before and during trial revealed a positive slope in ppFEV1 with a trend toward significance (Figure 3C; P = .060). There were no significant differences in demographics, baseline PFT, time from HCT to BOS, underlying HCT indications, and cGHVD characteristics between responders and nonresponders (Table 3).
PFT trajectory improves on pirfenidone. This figure illustrates the changes in spirometry metrics over time, comparing data from 24 months before the trial to measurements after starting pirfenidone. Red circles represent pretreatment values, and blue circles represent values while on pirfenidone. A regression line is fitted to the aggregate data, with the gray band indicating the 95% confidence interval. (A) ppFEV1 over time for all participants on pirfenidone (P = .00267). (B) ppFVC over time for all participants on pirfenidone (P = .170). (C) ppFEV1 over time for responders to pirfenidone (P = .060). (D) ppFVC over time for responders to pirfenidone (P = .122). Regression line equations display intercept and slope.
PFT trajectory improves on pirfenidone. This figure illustrates the changes in spirometry metrics over time, comparing data from 24 months before the trial to measurements after starting pirfenidone. Red circles represent pretreatment values, and blue circles represent values while on pirfenidone. A regression line is fitted to the aggregate data, with the gray band indicating the 95% confidence interval. (A) ppFEV1 over time for all participants on pirfenidone (P = .00267). (B) ppFVC over time for all participants on pirfenidone (P = .170). (C) ppFEV1 over time for responders to pirfenidone (P = .060). (D) ppFVC over time for responders to pirfenidone (P = .122). Regression line equations display intercept and slope.
Assessment of immune modulation, infection, and cGVHD flare on pulmonary function
To assess factors affecting PFTs, we compared the change in ppFEV1 and ppFVC from enrollment to completion of the 12-month trial for the following: respiratory infection and immune modulation (prednisone, ruxolitinib, or belumosudil). Mean prednisone dose for participants at enrollment and completion of the 12-month trial was 8.5 mg (standard deviation [SD], 9.0) and 7.6 mg (SD, 7.2), respectively. The relationship between PFT and average daily prednisone dose was not significant for ppFEV1 (∣r∣ < 0.3; P = .80) or ppFVC (∣r∣ < 0.3; P = .70). Treatment with belumosudil or ruxolitinib also was not associated with changes in PFT. Mean cumulative prednisone dose in participants with infection of 3900 mg (SD, 2274) vs without infection 2275 mg (SD, 2093) was not significantly different (P = .107). Participants with documented infection had a greater decrease in PFT during the study period (ppFEV1, –6% [SD, 8.6]; ppFVC, −11% [SD, 8.2]) compared with those without infection (ppFEV1 –0.7% [SD, 4.5], ppFVC −1.9% [SD, 8.7]), showing statistical significance between the groups for ppFEV1 (P = .045) and ppFVC (P = .03). To assess the association of prednisone exposure and infection with response to pirfenidone, we analyzed differences for responders vs nonresponders. The mean daily prednisone dose was 8.8 mg/day (SD, 5.7 mg) and 7.6 mg/day (SD, 8.7), respectively, and showed no statistically significant difference between the groups (P = .674). Infection density was 0.34 per year (SD, 0.40) and 0.25 per year (SD, 0.42), respectively, also without significant difference between groups (P = .562). Infection type and pathogen by participant are listed in supplemental Table 2. These results suggest that prednisone did not confound the therapeutic benefit of pirfenidone for BOS, and while infection was associated with decreased PFT, response to pirfenidone was not confounded by infection.
Responder analysis by qCT
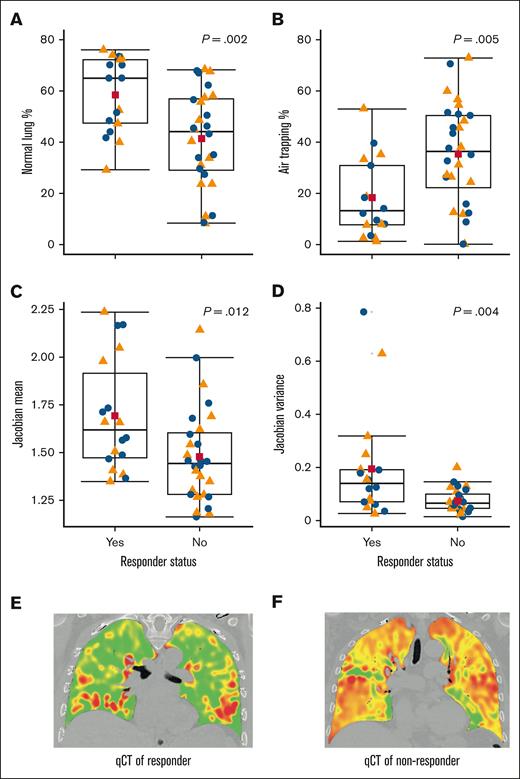
To evaluate if PFT improvements could be assessed radiographically, we obtained qCT scans for 23 participants at enrollment. These scans were analyzed for percentage normal lung, percentage air trapping, Jacobian index, and Jacobian variance, both for whole-lung and by lobe. Whole-lung analysis showed no significant differences between responders and nonresponders. However, significant differences were observed in the upper lobes. Responders had healthier lungs, indicated by higher percentage normal lung (61.2% vs 41.0%; P = .002), lower percentage air trapping (16.5% vs 35.2%; P = .005), greater lung volume change (Jacobian 1.73 vs 1.47; P = .012), and greater heterogeneity of lung volume change (Jacobian variance 0.21 vs 0.07; P = .004). Box plots in Figure 4 illustrate these distributions. Given no significant differences in baseline PFT between responders and nonresponders, these results suggest that qCT may be a valuable tool beyond PFT for identifying potential responders to pirfenidone.
Responders to pirfenidone have qCT metrics in the upper lobes that are consistent with healthier lungs. This figure presents qCT metrics for the upper lobes of 23 patients, divided into 9 responders and 14 nonresponders. (A) Percentage of normal lung. (B) Percentage of air trapping. (C) Mean Jacobian. (D) Variance of Jacobian. (E) Representative qCT coronal cut of responder. (F) Representative qCT coronal cut of nonresponder. Orange triangles represent the right upper lobe; blue dots represent the left upper lobe; red squares indicate the mean values; horizontal lines show the median values, with the IQR represented by the corresponding box. All qCT metrics show statistically significant differences between responders and nonresponders. The P values, calculated using the Wilcoxon rank-sum test, are displayed per panel.
Responders to pirfenidone have qCT metrics in the upper lobes that are consistent with healthier lungs. This figure presents qCT metrics for the upper lobes of 23 patients, divided into 9 responders and 14 nonresponders. (A) Percentage of normal lung. (B) Percentage of air trapping. (C) Mean Jacobian. (D) Variance of Jacobian. (E) Representative qCT coronal cut of responder. (F) Representative qCT coronal cut of nonresponder. Orange triangles represent the right upper lobe; blue dots represent the left upper lobe; red squares indicate the mean values; horizontal lines show the median values, with the IQR represented by the corresponding box. All qCT metrics show statistically significant differences between responders and nonresponders. The P values, calculated using the Wilcoxon rank-sum test, are displayed per panel.
To determine qCT thresholds that stratify risk, we conducted a decision tree analysis using CIT with upper lobe qCT metrics as covariates and response to pirfenidone as its outcome. Jacobian variance had the strongest discrimination value. The decision tree identified a high Jacobian variance (>0.082) as indicative of responders (6 responders, 3 nonresponders), and a low Jacobian variance (≤0.082) as indicative of nonresponders (3 responders, 11 nonresponders; P = .009). This threshold identified 79% of nonresponders (n = 11/14). Together these results suggest that intra-lung heterogeneity in volume expansion (ie, greater Jacobian variance) was a strong discriminating factor for those who would respond to treatment with pirfenidone.
PROs and cGVHD indices
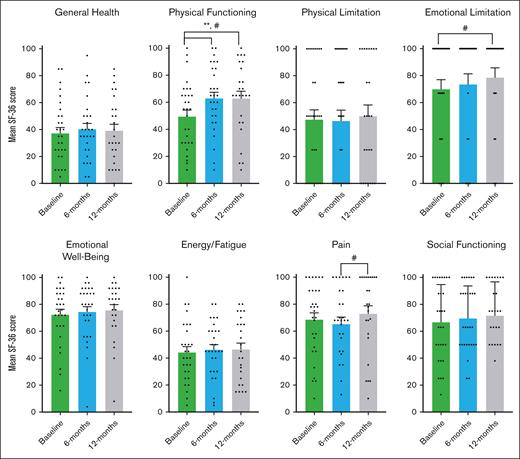
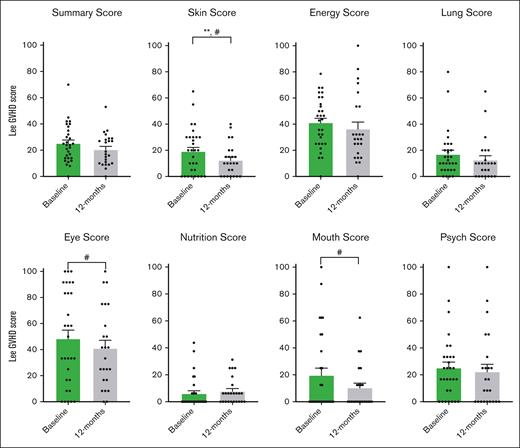
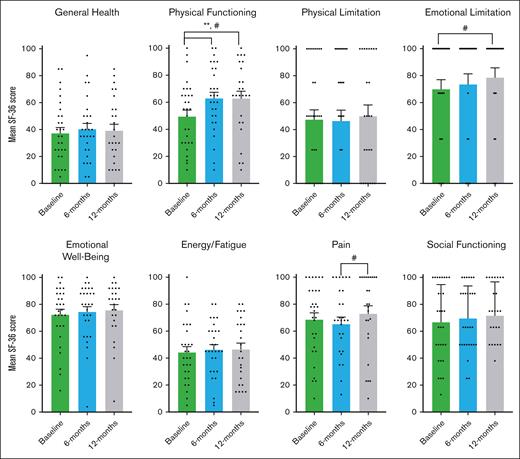
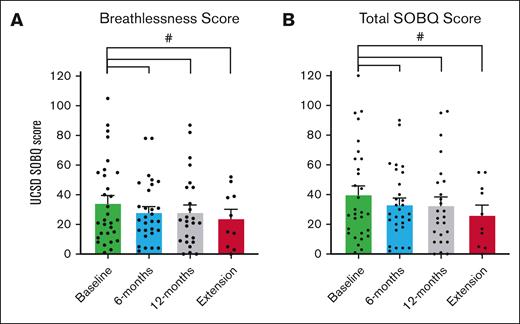
Participants reported improvements in physical functioning, shortness of breath, and cGVHD symptoms using 2 PRO study scales and the LSS cGVHD. During pirfenidone treatment, the SF-36 showed significant improvement in physical functioning, achieving MCID, and statistical significance from baseline to 6 months (11.65 points; P = .012), and from baseline to 12 months (8.48 points; P = .041; Figure 5). When analyzed by pirfenidone response status, this significance persisted in the responder group but not in the nonresponders (supplemental Table 3). Improvements were also noted in emotional limitations from baseline to 12 months, and in pain from 6 to 12 months (Figure 5). For the UCSD SOBQ, there was a reduction achieving MCID in breathlessness and total SOBQ score from baseline to 6 months, 12 months, and through the extension period (Figure 6). Using LSS (Figure 7), we observed a clinically and statistically significant improvement in skin cGVHD symptoms after 12 months of pirfenidone treatment (P = .007), along with MCID improvements in eye and mouth symptoms.
SF-36 health survey scores in pirfenidone trial. This figure shows the results of the Medical Outcomes Study SF-36 Health Survey Questionnaire at baseline, 6 months, and 12 months during the Safety and Tolerability of Pirfenidone for Bronchiolitis Obliterans Syndrome trial. The SF-36 survery includes 36 items that assess quality of life across 8 areas: general health perception, physical health problems, role limitations due to physical or emotional problems, emotional well-being, energy/fatigue, pain, and social functioning. Scores range from 0 to 100, with higher scores indicating better health. An increase of more than 5 points is considered a MCID, denoted by #. The data presented are the mean SF-36 percentage scores and the standard error of the mean, analyzed using a paired t test. Statistically significant differences (P < .05) are indicated by ∗∗.
SF-36 health survey scores in pirfenidone trial. This figure shows the results of the Medical Outcomes Study SF-36 Health Survey Questionnaire at baseline, 6 months, and 12 months during the Safety and Tolerability of Pirfenidone for Bronchiolitis Obliterans Syndrome trial. The SF-36 survery includes 36 items that assess quality of life across 8 areas: general health perception, physical health problems, role limitations due to physical or emotional problems, emotional well-being, energy/fatigue, pain, and social functioning. Scores range from 0 to 100, with higher scores indicating better health. An increase of more than 5 points is considered a MCID, denoted by #. The data presented are the mean SF-36 percentage scores and the standard error of the mean, analyzed using a paired t test. Statistically significant differences (P < .05) are indicated by ∗∗.
UCSD SOBQ scores in pirfenidone trial and during extension trial. This figure presents the UCSD SOBQ scores at baseline, 6 months, 12 months, and during the extension phase (12-56 months) of the Safety and Tolerability of Pirfenidone for Bronchiolitis Obliterans Syndrome trial. The UCSD SOBQ consists of 24 questions, each scored on a scale from 0 to 5, with total scores ranging from 0 to 120. Higher scores indicate more severe shortness of breath. (A) Breathlessness score: assessed by 21 questions focusing on the severity of shortness of breath during activities of daily living. (B) Total shortness of breath score: includes the breathlessness score plus 3 additional questions that evaluate fear related to limitations. The data are presented as mean scores with the standard error of the mean. A MCID, denoted by #, is defined as a reduction of >5 points from baseline.
UCSD SOBQ scores in pirfenidone trial and during extension trial. This figure presents the UCSD SOBQ scores at baseline, 6 months, 12 months, and during the extension phase (12-56 months) of the Safety and Tolerability of Pirfenidone for Bronchiolitis Obliterans Syndrome trial. The UCSD SOBQ consists of 24 questions, each scored on a scale from 0 to 5, with total scores ranging from 0 to 120. Higher scores indicate more severe shortness of breath. (A) Breathlessness score: assessed by 21 questions focusing on the severity of shortness of breath during activities of daily living. (B) Total shortness of breath score: includes the breathlessness score plus 3 additional questions that evaluate fear related to limitations. The data are presented as mean scores with the standard error of the mean. A MCID, denoted by #, is defined as a reduction of >5 points from baseline.
LSS in pirfenidone trial. This figure shows the results of the LSS, which assess symptoms associated with cGVHD across 7 domains: skin, energy, lung, eye, nutrition, mouth, and psychological well-being. The questionnaire contains 30 items, each rated on a 5-point Likert scale. Scores range from 0 to 100, with higher scores indicating more severe symptoms of cGVHD. MCID: a change of more than 5 points is considered clinically significant, denoted by #. Questionnaires were administered at baseline and 12 months after pirfenidone treatment. The data are presented as mean scores with the standard error of the mean, and analyzed for significance using paired t tests. Statistically significant differences (P < .05) are indicated by ∗∗. Psych, psychological.
LSS in pirfenidone trial. This figure shows the results of the LSS, which assess symptoms associated with cGVHD across 7 domains: skin, energy, lung, eye, nutrition, mouth, and psychological well-being. The questionnaire contains 30 items, each rated on a 5-point Likert scale. Scores range from 0 to 100, with higher scores indicating more severe symptoms of cGVHD. MCID: a change of more than 5 points is considered clinically significant, denoted by #. Questionnaires were administered at baseline and 12 months after pirfenidone treatment. The data are presented as mean scores with the standard error of the mean, and analyzed for significance using paired t tests. Statistically significant differences (P < .05) are indicated by ∗∗. Psych, psychological.
Discussion
In this 56-week open-label study with a 56-month extension, we found that pirfenidone was safe and generally well tolerated in patients with BOS following HCT. Forty-one percent of participants demonstrated improved ppFEV1 and/or ppFVC trajectories during treatment, with overall stabilization in ppFEV1 compared with pretreatment baseline. Given the number of participants we found to be responders to pirfenidone in the interim analysis,13 we considered it imperative to continue therapy for a disease that lacks alternative proven treatment options. The extension trial afforded us a platform to extend therapy despite off-label use, and to observe the multiyear feasibility and impact of pirfenidone on a vulnerable population. Additionally, qCT at enrollment provided a strong signal in stratifying responders before treatment, a novel finding beyond the capacity of PFT to develop clinical trial inclusion criteria, and improved monitoring strategies for BOS. These results are encouraging given the significant morbidity and mortality associated with BOS following allogeneic HCT.
Our study is the first to evaluate pirfenidone prospectively for the treatment of BOS after HCT. In patients with IPF, pirfenidone can cause dose-limiting AEs such as fatigue, GI discomfort, and a photosensitive rash.10 A recent randomized controlled trial in patients with CLAD also demonstrated treatment tolerability of pirfenidone.23 In our study, 63% of participants tolerated the recommended dose, similar to a post-authorization safety study in IPF where 46% of patients required dose reductions.24 Fatigue and GI symptoms were frequently reported by participants, with notable overlap between pirfenidone-related symptoms and those associated with cGVHD.25-27 Fatigue was reported by 83.3% of participants, comparable to 84% in previous cGVHD reports.28,29 Among allogeneic HCT recipients with suspected GI cGVHD, symptoms included diarrhea (74%), nausea/vomiting (33%), weight loss (19%), and gastroesophageal reflux disorder (5%).30 Treatment of IPF with pirfenidone has also been linked to increased GI-related adverse reactions, including nausea, diarrhea, and weight loss.31
In our cohort, baseline LFTs prior to trial enrollment were elevated, consistent with underlying cGVHD. After 12 months of treatment, we observed a significant decrease in all LFTs, which was sustained in the 10 participants who continued through the 56-month extension. However, the clinical significance of this decrease is uncertain. Liver dysfunction occurs in up to 50% of patients with cGVHD, with the exact mechanism being incompletely understood.32 Indolent elevations of ALT and ALP are early indicators of hepatic cGVHD, but isolated liver enzyme elevations are not diagnostic of cGVHD and likely reflect systemic cGVHD activity.32 Although hepatoxicity with pirfenidone is rare, pooled analysis of 3 large trials showed LFT elevations (>3 times upper limit of normal) in 3.2% of patients.33 Pirfenidone has nonetheless been shown to improve liver fibrosis biomarkers in patients with advanced liver disease. Given that cGVHD of the liver is thought to be a progressive transforming growth factor-β driven process,34 a positive effect of pirfenidone is biologically plausible.
Evaluations of drug interactions and infections were important considerations in our analysis. Concurrent SOC therapies during the study period allowed for evaluation of interactions. Although we found that infection was associated with a decline in PFT, similar rates of prednisone use and infection regardless of responder status suggest that pirfenidone response was not confounded by prednisone use or infection. This is notable, given that a prior study on infections and mortality in patients with BOS found that 61.8% of persons with BOS had at least 1 respiratory tract infection, and of this group, a total of 82.4% had recurrent infection.35 Those with infection had lower overall survival to an extent that achieved statistical significance.
qCT with lobe segmentation revealed that patients responding to pirfenidone have metrics consistent with healthier lungs at baseline, especially in the upper lobes. Nonresponders exhibited a markedly narrow IQR for Jacobian variance (Figure 4D). CIT analysis revealed that high Jacobian variance, reflecting heterogeneity of volume expansion, could be used to screen for pirfenidone response. Low heterogeneity of lung volume expansion (ie, low Jacobian variance) suggests a more global reduction in ventilation for nonresponders compared with responders, which is a marker of severity and poor response to therapy in other obstructive airways diseases.36-38 Responders may be in earlier stages of their disease, with less fixed airway fibrosis typical of advanced disease. Studies using quantitative imaging with xenon magnetic resonance imaging have found that obstructive airway diseases often exhibit greater ventilation abnormalities in the upper lobes.39,40 BOS may begin at the lung bases and progress upward, similar to the progression seen in other fibrotic diseases like IPF.41 Patients with healthier upper lobes (West zone 1) maintain better ventilation, potentially showing more significant improvements in PFT trajectories with appropriate therapy.42 Dependent atelectasis of the lung bases can complicate patient stratification using a global metric like PFT, making lobar segmentation with qCT more useful. Notably, a recent study found no significant response to pirfenidone as measured by qCT in individuals with CLAD.23 In addition to not meeting recruitment goals due to the COVID-19 pandemic, the study’s negative finding may have been due to lack of lobar segmentation with qCT. Overall, our findings imply that qCT offers valuable stratification capabilities beyond the current capacity of PFT, particularly with the detailed insights provided by regional radiographic analysis.
Given the morbidity and impaired quality of life associated with BOS, improvement in PROs was a notable finding in our study. Significant improvements were observed in the SF-36 physical functioning score at 6 and 12 months. Unlike the interim analysis, which showed significance at 6 months only, this full analysis shows significance at 12 months,13 which aligns with observations in individuals treated with pirfenidone for IPF.43 Using the well-validated LSS, we also observed improvements in cGVHD-associated skin, oral, and ocular symptoms, consistent with our interim study results.
This study has limitations. First, it lacked a control arm, as patients were compared with previous PFT values obtained up to 24 months prior to treatment and, thus, no definitive efficacy conclusions can be drawn from this study design. Second, the study was not statistically powered to determine the efficacy of pirfenidone for treating BOS after HCT, and pretreatment lung function data were limited. Instead, the study was powered to assess the tolerability of the recommended pirfenidone dose. We set an a priori tolerability threshold of >25% to justify proceeding with a future randomized trial. However, the study design limits the generalizability of our findings and introduces potential participant and clinical bias. Third, this was a single-center study, which limits external validity. Future multicenter trials are needed. Fourth, the small number of participants in this trial for a rare disease is a limitation. We addressed this by collection of a rich data set over 56 months, including PFT trajectory, laboratory tests, and PROs to characterize disease progression, and represent relevant physiology and multiple dimensions of the patient experience. Finally, the median time between BOS diagnosis and trial enrollment (28.5 months, [IQR, 13.9-56.8]) suggests that some patients may already have had fixed and nonmodifiable disease prior to enrollment, with the active cGVHD process in the lungs already being quiescent before receiving pirfenidone. This would limit the assessment of drug utility. Notably, subgroup analysis by responder status did not show a statistically significant difference in time between HCT or diagnosis and trial enrollment (P = .24 and P = .62, respectively, Table 3).
In our single-center, open-label pilot study, we showed pirfenidone’s acceptable tolerability and potential to attenuate the progression of BOS. Significant improvement observed in extrapulmonary cGVHD symptoms also suggests pirfenidone not only as a viable treatment option for BOS, but also as a potential novel treatment for cGVHD. Despite sample size limitations, the data presented and the putative clinical impact of pirfenidone are intriguing clinically and scientifically. These findings support targeting systemic fibrosis for cGVHD and provide justification for a randomized controlled trial to test pirfenidone’s efficacy in BOS after HCT.
Acknowledgments
The authors thank all patients for their willingness to participate in the study. The authors also thank Guy Livneh for his advocacy for patients with BOS and support of the Stanford Lung GVHD clinic.
J.L.H. was funded by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) grants K08HL122528-01A1 and R01HL157414-01 during the study period. G.-S.C. is funded by grants P30 CA015704 from the NIH/National Cancer Institute, R01HL161037 from the NIH/NHLBI, and a grant from the V-Foundation. Genentech, Inc (South San Francisco, CA) provided drug support for the trial. The Blood and Marrow Transplant database is funded by NIH grants P01 CA049605 and P01 HL075462.
Authorship
Contribution: J.L.H. conceived and designed the study; H.S., C.T.M., Z.M., C.H.B., E.I.M., C.O., L.J., and J.L.H. collected the data; H.S., E.I.M., C.T.M., C.O., A.B., C.G., G.-S.C., and J.L.H. performed the data analysis and interpretation; H.S., C.T.M., Z.M., C.H.B., A.S., G.Y., G.-S.C., and J.L.H. drafted the manuscript; H.S., C.T.M., Z.M., C.H.B., C.O., G.-S.C., and J.L.H. verified the data; and all authors contributed to revisions of the manuscript, and read and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Husham Sharifi, Division of Pulmonary, Allergy and Critical Care Medicine, Stanford University School of Medicine, 300 Pasteur Dr, Stanford, CA 94305-5236; email: husham@stanford.edu.
References
Author notes
An interim analysis of the Safety and Tolerability of Pirfenidone for Bronchiolitis Obliterans Syndrome trial was published in Bone Marrow Transplantation 2022, and presented in abstract form at the 115th American Thoracic Society Conference, 7 August 2020 (virtual). A version of this study was also presented in abstract form in the 120th American Thoracic Society Conference, San Francisco, CA, 18 May 2025.
Datasets for the trial can be shared in fully deidentified form upon request to the corresponding author, Husham Sharifi (husham@stanford.edu) and with the consent and evaluation of Stanford Institutional Review Board.
The full-text version of this article contains a data supplement.