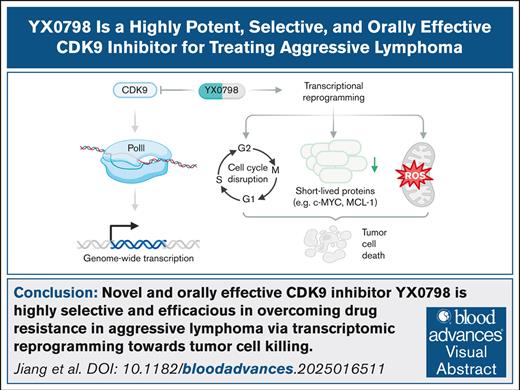
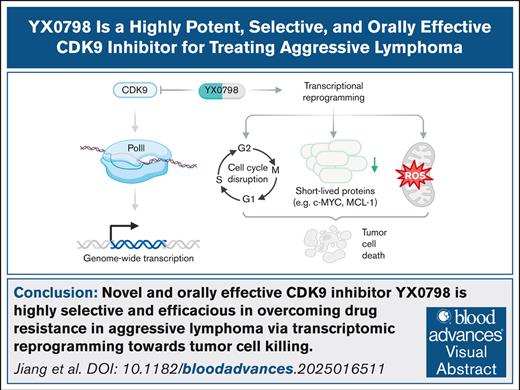
Key Points
Novel CDK9i YX0798 showed high selectivity and antitumor efficacy in overcoming drug resistance in aggressive lymphoma.
YX0798 had good oral pharmacokinetics and antitumor effects that resulted from transcription reprogramming toward tumor cell killing.
Visual Abstract
Nongenetic transcription evolution has been increasingly explored and recognized to drive tumor cell progression and therapeutic resistance. As the regulation hub of transcription machinery, cyclin-dependent kinase 9 (CDK9) is the gatekeeper of RNA polymerase II transcription, and CDK9 dysfunction results in transcriptomic reprogramming and tumor cell progression. We recently reported that the heat shock protein 90 (HSP90)-MYC-CDK9 network drives therapeutic resistance in mantle cell lymphoma (MCL) through transcriptomic reprogramming. We also showed that targeting CDK9 by AZD4573 and enitociclib is a safe and effective treatment in preclinical Mantle Cell Lymphoma (MCL) models, supporting CDK9 as a valid therapeutic target for MCL. However, current CDK9 inhibitors (CDK9is) under therapeutic development have room for improvement due to limited target selectivity and oral bioavailability. To this end, YX0798 was discovered to be a novel CDK9i through structural optimization. YX0798 demonstrated remarkable target selectivity and high affinity in binding to CDK9. Furthermore, YX0798 showed good oral bioavailability. YX0798, when administrated orally (5 mg/kg daily), led to an efficacious antitumor activity in vivo and showed the potency in overcoming therapeutic resistance. Mechanistically, YX0798 downregulated the short-lived oncoprotein c-MYC and prosurvival protein myeloid cell leukemia sequence 1 (MCL-1) as a common mechanism of CDK9 inhibition. Moreover, YX0798 disrupted the cell cycle and resulted in transcriptomic reprogramming, eventually leading to cell death. Furthermore, YX0798 has the potential to be used in combination therapy with clinical agents to improve treatment efficacy. Together, these data demonstrate that YX0798 has oral bioavailability, exquisite selectivity, and antitumor potency that results from driving transcription reprogramming toward tumor cell killing.
Introduction
Nongenetic transcription reprogramming has been recognized to be a major driver of tumor progression and therapeutic resistance.1-3 Cyclin-dependent kinase 9 (CDK9) is a central regulator of RNA polymerase II (Pol II), which transcribes all protein-coding genes and many noncoding RNAs.4 CDK9 forms a heterodimer with cyclin T1 or T2 to form the positive transcription elongation factor b (p-TEFb), which phosphorylates Pol II to trigger the release of a paused Pol II and resume transcription.5 In addition, CDK9 also mediates Pol II transcription initiation and termination.6-8 Transcriptional dysfunction results in transcriptomic reprogramming and promotes cancer development; therefore, targeting CDK9 has the potential to overcome therapeutic resistance.9 In fact, CDK9 has been recognized as a therapeutic target in treating cancer and a few CDK9 inhibitors (CDK9is) are under clinical investigation.10,11 Recently, for mantle cell lymphoma (MCL), an aggressive subtype of non-Hodgkin lymphoma, we and others have reported that transcriptomic reprogramming is strongly associated with disease progression and therapeutic resistance.12-14 For patients with MCL, next-generation sequencing showed that CDK9 expression was upregulated, and the cancer hallmark MYC_TARGETS was highly enriched in individuals who relapsed after chimeric antigen receptor (CAR) T-cell therapy, suggesting that targeting CDK9 could overcome that resistance.15
As CDK9 has long been recognized as a therapeutic target, small molecules have been generated to inhibit CDK9; however, many have failed in clinical trials due to a lack of target selectivity and indiscriminate binding with other CDKs.10 To improve this, more selective CDK9is, such as AZD457316 and enitociclib (also known as VIP152),17,18 have been developed and shown to be effective in hematologic malignancy models, including in MCL preclinical models developed by our group.15,19 These CDK9is were advanced to phase 1/2 clinical investigation but showed limited treatment efficacy due to toxicity.11 In addition, both CDK9is are administrated by IV infusion, and no oral bioavailability has been reported. In this study, we discovered YX0798 as a potent, selective, and safe CDK9i with oral bioavailability that could be beneficial for treating cancer and improving patient outcomes.
Methods
Chemicals
YX0798 was designed and synthesized through structure-based rational drug design through multiple rounds of optimization. The synthesis of YX0798 and its structural analogs (n > 70) will be reported in a separate study. A provisional patent application has been filed to protect the intellectual property of YX0798 and its analogs. Other compounds, enitociclib, AZD4573, ibrutinib, zanubrutinib, pirtobrutinib, safimaltib, AZD4320, and NX-2127 were purchased from MedChemExpress. The compounds were prepared in dimethyl sulfoxide (DMSO) for in vitro studies, and in 2% DMSO, 30% polyethylene glycol 400, and 5% Tween-80 for in vivo studies.
3D molecular docking
Molecular docking was performed with Small Molecule Drug Discovery Suite (Schrödinger release 2022-2; Schrödinger, LLC, New York, NY, 2019).20,21 The cocrystal of CDK9 with AZD4573 (Protein Data Bank [PDB] ID: 6Z45) was obtained from Research Collaboratory for Structural Bioinformatics PDB. The selected structure was preprocessed and optimized with Schrödinger Protein Preparation Wizard. The 20-Å grid box was generated using Glide Grid and centered on AZD4573. Compound YX0798 was created with Maestro and prepared with LigPrep to generate a suitable 3-dimensional (3D) conformation for docking. Ligand docking was performed with Glide using SP mode with default settings. The docked pose was incorporated into Maestro pose viewer to visualize the interaction of the binding site.
Kinome profiling and binding affinity
Kinome profiling of YX0798 at 100 nM was performed using the scanMAX assay (Eurofins) against a panel of 468 kinases and relevant mutants. Kinase selectivity of YX0798 was visualized using TREEspot software (Eurofins). The binding affinity of YX0798 and AZD4573 to CDK9 was determined using the kdELECT assay (Eurofins).
Cell lines
MCL cell lines JeKo-1, JeKo-R (with acquired ibrutinib resistance), JeKo BTK KD (with intrinsic ibrutinib resistance due to BTK depletion), Mino, Mino-R (with acquired resistance to venetoclax), Maver-1, and Z138 were authenticated by single-nucleotide polymorphism profiling and maintained as described previously.22,23
Patient samples
Patient samples were collected from peripheral blood or apheresis after obtaining written informed consent and approval from the institutional review board at The University of Texas MD Anderson Cancer Center. Peripheral blood mononuclear cells from healthy donors were purchased from the Gulf Coast Regional Blood Center.
Cell viability, cell cycle, apoptosis, and western blotting
These assays were performed as described previously.12 Antibodies used to visualize proteins are shown in supplemental Table 1.
Measurement of cellular oxidative stress
Cellular reactive oxygen species (ROS) levels were measured using CellROX Green Reagent (5 μM; catalog no. C10444; Invitrogen) according to the manufacturer’s manual.
Efficacy evaluation in PDO models
This assay was performed as described previously.12 Briefly, the patient-derived organoid (PDO) models were established using primary patient samples. The PDO models were treated with BTK inhibitors (BTKis) or CDK9is for 72 hours and cell viability was measured using CellTiter-Glo 3D assay kits (Promega, G9681) following the manufacturer’s protocol.
In vivo efficacy assessment in PDX models
These assays were performed as described previously.12 The institutional animal care and use committee of The University of Texas MD Anderson Cancer Center approved the experimental protocols involving animals. Briefly, patient-derived xenograft (PDX) models were established by IV inoculating MCL cells into NOD.Cg-Prkdc scid Il2rg tm1Wjl /SzJ (ie, NSG) mice. When the tumors became palpable, mice (n = 5 for each group) were treated with compounds. Tumor volumes, survival duration, and body weight were monitored. Tumor volumes were calculated as volume = (length × width × width)/2.
Metabolic and pharmacokinetic profile studies of YX0798
The liver microsome stability assay, the MDCK-MDR1 permeability assay, kinetics solubility determination, and the in vivo pharmacokinetics study of YX0798 were performed by a contract research organization (CRO) service (BioDuro-Sundia, Irvine, CA).
Bulk RNA sequencing
The bulk RNA sequencing and analysis were performed as described previously.15
Statistical analysis
All statistical analyses were conducted using R software (version 4.0.3) and GraphPad Prism (version 9). A 2-sided 2-sample t test was used to compare differences between groups. Statistical significance was indicated as follows: ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001.
Results
YX0798 is a novel CDK9i with higher target selectivity than AZD4573
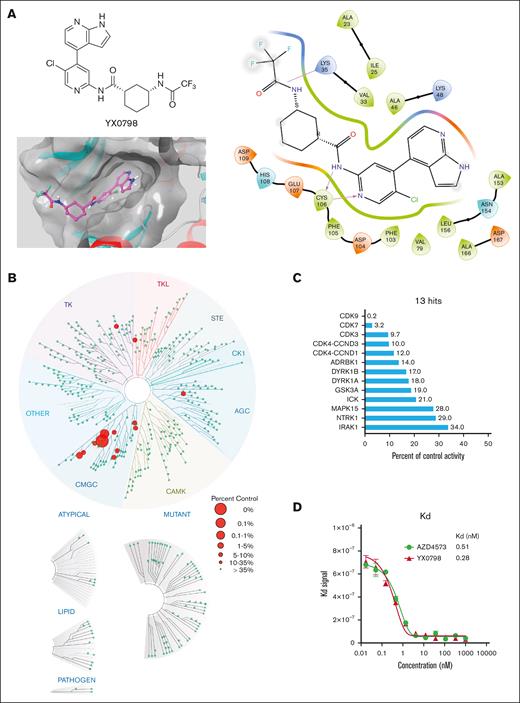
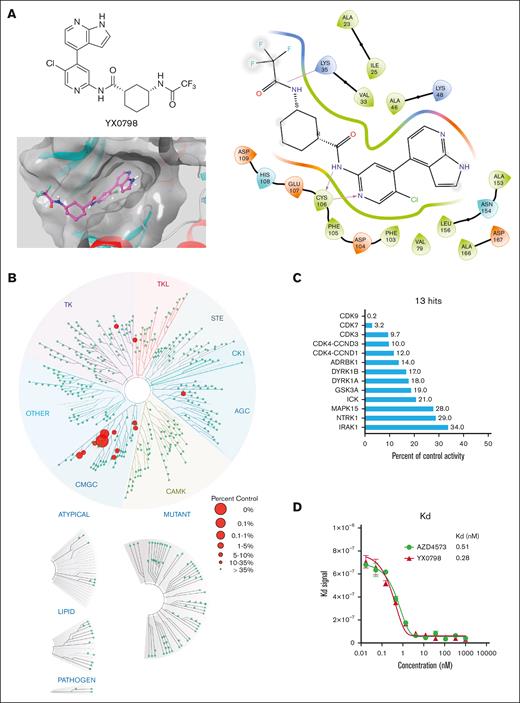
To develop CDK9i candidates with high selectivity and oral bioavailability, we designed and generated >70 CDK9is (data not shown) based on CDK9i AZD4573 through multiple rounds of structural optimization. YX0798 (Figure 1A) was identified as the most potent CDK9i candidate based on antitumor efficacy screening in vitro. To visualize how YX0798 binds to CDK9, we performed YX0798-CDK9 molecular docking via Schrödinger using a AZD4573-CDK9 cocrystal structure (PDB: 6Z45). Similar to AZD4573, YX0798 binds to CDK9 in the adenosine triphosphate–binding site of CDK9. YX0798 bound well to the CDK9 active site,24 including a hinge moiety and binding moiety deep in the pocket. The aminopyridine skeleton formed crucial dual hydrogen bonds with Cys106, which contributed to the high affinity of YX0798 with CDK9 (Figure 1A, right). Other key interactions, such as the hydrophobic interaction of the protein with the trifluoromethyl group, also contributed to the selective binding.
Identification of YX0798, a potent and selective CDK9i. (A) Chemical structure of YX0798 (upper left) and YX0798-CDK9 molecular docking (bottom left). YX0798-CDK9 molecular docking via Schrödinger, using a publicly available AZD4573-CDK9 cocrystal structure. YX0798 interaction with CDK9 visualized in the adenosine triphosphate–binding pocket (right). (B) Kinome profiling of YX0798 at 100 nM using a scanMAX panel consisting of 468 kinases (Eurofins) and (C) the 13 hits identified with > 66% kinase inhibition. (D) The binding affinity of CDK9i YX0798 and AZD4573 using a kdELECT assay (Eurofins).
Identification of YX0798, a potent and selective CDK9i. (A) Chemical structure of YX0798 (upper left) and YX0798-CDK9 molecular docking (bottom left). YX0798-CDK9 molecular docking via Schrödinger, using a publicly available AZD4573-CDK9 cocrystal structure. YX0798 interaction with CDK9 visualized in the adenosine triphosphate–binding pocket (right). (B) Kinome profiling of YX0798 at 100 nM using a scanMAX panel consisting of 468 kinases (Eurofins) and (C) the 13 hits identified with > 66% kinase inhibition. (D) The binding affinity of CDK9i YX0798 and AZD4573 using a kdELECT assay (Eurofins).
To determine the selectivity of YX0798, we performed kinome profiling using the scanMAX assay, which includes a panel of 468 kinases. At 100 nM, YX0798 led to only 13 hits with reduced activity ≥66% (Figure 1B). Specifically, YX0798 led to a 99.8% reduction against CDK9 activity, which is 16 to 50-fold more selective for CDK9 than CDK7, CDK3, or CDK4 (Figure 1C). In contrast, AZD4573 was reported to have 16 hits with reduced binding ≥90%.16 Furthermore, YX0798 showed an extremely high binding affinity (dissociation constant (Kd)= 0.28 nM; Figure 1D), which was 357-fold lower than the concentration used for the kinome profiling (Figure 1C), whereas AZD4573 showed a relatively weaker affinity (Kd = 0.51 nM; Figure 1D). These results confirm that YX0798 has a greater CDK9 affinity and selectivity than AZD4573.
YX0798 inhibits CDK9 activity and potently kills MCL cells in vitro
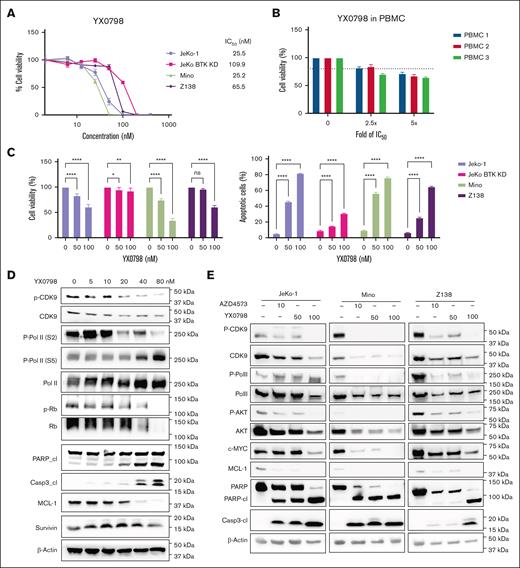
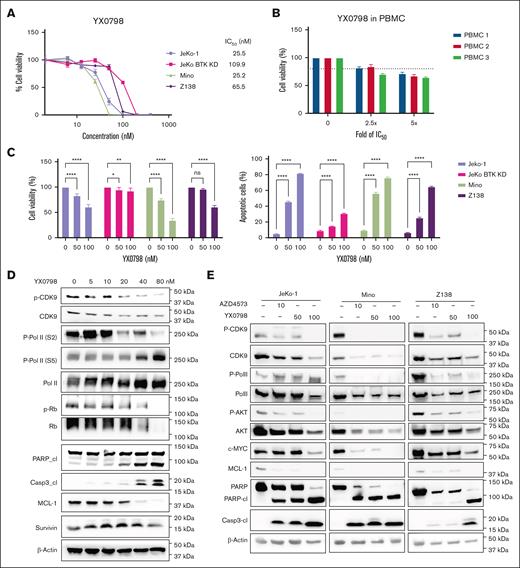
YX0798 (50% inhibitory concentration (IC₅₀), 25.2-109.9 nM) was highly potent in killing BTKi-sensitive MCL cells (Mino and JeKo-1) and BTKi-resistant cells (JeKo BTK KD and Z138; Figure 2A), but not healthy peripheral blood mononuclear cells (Figure 2B). AZD4573 was more potent than YX0798 in killing MCL cells with a lower IC₅₀ (6.5-13.2 nM; supplemental Figure 1A-B). Similar to AZD4573, YX0798 potently inhibited cell viability and induced apoptosis in all MCL cell lines assessed (Figure 2C; supplemental Figure 1C). YX0798 inhibited CDK9 phosphorylation, induced poly-adenosine diphosphate (ADP) ribose polymerase (PARP) and caspase 3 cleavage, and reduced antiapoptotic MCL-1 in a dose-dependent manner in Z138 cells (Figure 2D). YX0798 also dose-dependently suppressed phosphorylation of CDK9 substrate RNA Pol II at Ser2, but not at Ser5. As a CDK4/6 downstream signaling event, phosphorylation of retinoblastoma protein was not inhibited upon treatment with YX0798 at low concentrations until it reached 80 nM, whereas total retinoblastoma protein was also reduced. Likewise, no dramatic changes of survivin, primarily expressed at the G2M phase, were detected until YX0798 reached 80 nM. These data support that YX0798 selectively inhibits CDK9, but not CDK7 or cell cycle CDKs, such as CDK4/6. The potency of YX0798 was also demonstrated in Mino and JeKo-1 cells (Figure 2E), which was similar to that of AZD4573. Furthermore, a reduction of phosphorylation of RNA Pol II and non-CDK9 substrate Ak strain transforming (AKT) was seen in MCL cells treated with YX0798 or AZD4573 (Figure 2E). In addition to MCL-1, c-MYC was reduced in a dose-dependent manner by YX0798 (Figure 2E), which was previously reported for AZD4573 and enitociclib in MCL16,17 and non-MCL hematopoietic cancer cells.15,19 Together, these data support a common mechanism of action for CDK9is, leading to depletion of short-lived oncoprotein c-MYC and prosurvival MCL-1.
YX0798 inhibits CDK9 activity and potently kills MCL cells. (A) YX0798 potently inhibited cell viability of MCL cells (n = 4) at 72 hours after treatment, but not PBMCs from healthy donors (n = 3). (B) The doses of YX0798 used to treat PBMCs were 2.5-fold (100 nM) and fivefold (200 nM) of the average IC50 value of JeKo-1, Mino, and Z138 cells. (C) YX0798 inhibited cell viability and induced apoptosis in a dose-dependent manner at 20 hours after treatment. (D) YX0798 treatment for 6 hours inhibited CDK9 phosphorylation and induced cl of PARP and caspase-3, as well as MCL-1 depletion in a dose-dependent manner. (E) The effects of YX0798 on protein profiling in CDK9 pathways and apoptosis pathways upon treatment for 6 hours. AZD4573 serves as a positive control. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. cl, cleavage; IC50, 50% inhibitory concentration; ns, not significant; p-CDK9; phosphorylated cyclin-dependent kinase 9; p-Rb, phosphorylated retinoblastoma protein; PBMC, peripheral blood mononuclear cell; Rb, retinoblastoma protein.
YX0798 inhibits CDK9 activity and potently kills MCL cells. (A) YX0798 potently inhibited cell viability of MCL cells (n = 4) at 72 hours after treatment, but not PBMCs from healthy donors (n = 3). (B) The doses of YX0798 used to treat PBMCs were 2.5-fold (100 nM) and fivefold (200 nM) of the average IC50 value of JeKo-1, Mino, and Z138 cells. (C) YX0798 inhibited cell viability and induced apoptosis in a dose-dependent manner at 20 hours after treatment. (D) YX0798 treatment for 6 hours inhibited CDK9 phosphorylation and induced cl of PARP and caspase-3, as well as MCL-1 depletion in a dose-dependent manner. (E) The effects of YX0798 on protein profiling in CDK9 pathways and apoptosis pathways upon treatment for 6 hours. AZD4573 serves as a positive control. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. cl, cleavage; IC50, 50% inhibitory concentration; ns, not significant; p-CDK9; phosphorylated cyclin-dependent kinase 9; p-Rb, phosphorylated retinoblastoma protein; PBMC, peripheral blood mononuclear cell; Rb, retinoblastoma protein.
YX0798 is stable, safe, and orally bioavailable
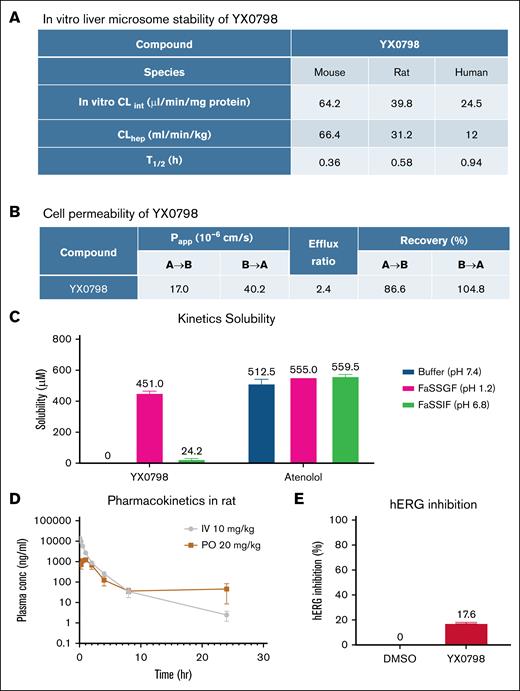
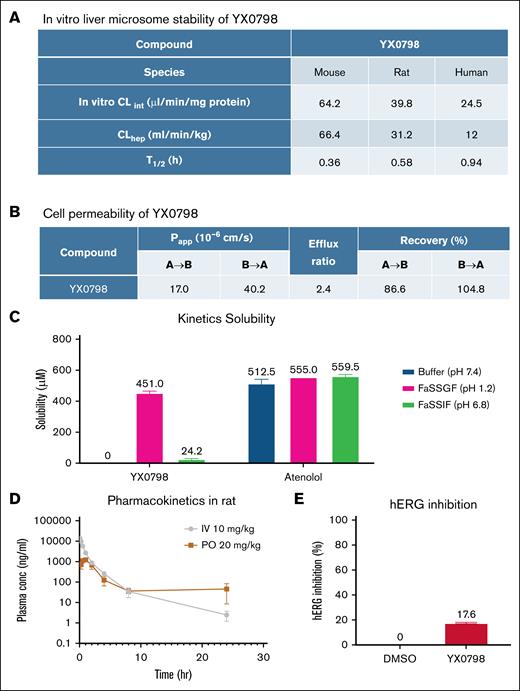
To further characterize the drug-like properties of YX0798, we performed in vitro liver microsome stability assays. YX0798 was stable with half-lives (T1/2) of 0.36, 0.58, and 0.94 hours in mouse, rat, and human, respectively (Figure 3A), which were comparable to AZD4573 (T1/2 of 0.18, 0.18, and 1.6 hours, respectively).24 YX0798 showed high cell permeability (Papp = 17.0 × 10–6 and 40.2 × 10–6 cm/s; efflux ratio, 2.4) as determined by the MDCK-MDR1 permeability assay (Figure 3B). YX0798 has high solubility (451 μM) in acidic fasted-state simulated gastric fluid buffer (pH 1.2), much lower solubility (24.2 μM) in fasted-state simulated intestinal fluid buffer (pH 6.8), and no detectable solubility in pH 7.4 buffer (Figure 3C). These data indicate that YX0798 is a basic drug given its pyridine core scaffold and high gastric fluid solubility. We next assessed the pharmacokinetic properties of YX0798 in rats following IV and oral administration. YX0798 has a lower clearance rate and longer T1/2 than those of AZD457324 (Figure 3D). Meanwhile, good plasma exposure after oral administration was seen, and the total drug exposure represented as the area under the curve across time (AUC0-∞) value for YX0798 was 4457 ng·h/mL. YX0798 oral bioavailability (F%) of YX0798 is 16.5%, which indicates its potential for oral administration, providing an advantage over other CDK9is such as AZD4573 and enitociclib with no oral bioavailability reported. The human ether-a-go-go-related gene (hERG) inhibition assay, used to evaluate the potential cardiotoxicity of a drug candidate, showed that YX0798 has weak hERG inhibition at 10 μM (Figure 3E), which is much higher than the highest plasma concentration (2.61 μM) the compound can reach by oral administration in rats. Therefore, we do not expect that YX0798 will cause cardiotoxicity. Together, these data indicate that YX0798 is a stable and safe CDK9i with acceptable oral bioavailability.
YX0798 shows oral bioavailability in rats. (A) YX0798 is metabolically stable in mouse, rat, and human as determined by an in vitro liver microsome stability assay. (B) YX0798 has high cell permeability. (C) YX0798 has good kinetic solubility in acidic FaSSGF. The beta-blocker atenolol was used as a control. (D) YX0798 showed oral bioavailability in rats. (E) YX0798 caused weak inhibition on hERG activity at 10 μM. CLhep, hepatic clearance; CLint, intrinsic clearance; FaSSGF, fasted-state simulated gastric fluid; FaSSIF, fasted-state simulated intestinal fluid; hERG, human ether-a-go-go related gene; PO, per os (by mouth).
YX0798 shows oral bioavailability in rats. (A) YX0798 is metabolically stable in mouse, rat, and human as determined by an in vitro liver microsome stability assay. (B) YX0798 has high cell permeability. (C) YX0798 has good kinetic solubility in acidic FaSSGF. The beta-blocker atenolol was used as a control. (D) YX0798 showed oral bioavailability in rats. (E) YX0798 caused weak inhibition on hERG activity at 10 μM. CLhep, hepatic clearance; CLint, intrinsic clearance; FaSSGF, fasted-state simulated gastric fluid; FaSSIF, fasted-state simulated intestinal fluid; hERG, human ether-a-go-go related gene; PO, per os (by mouth).
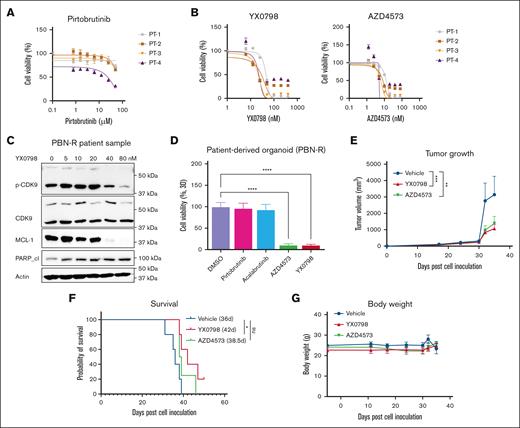
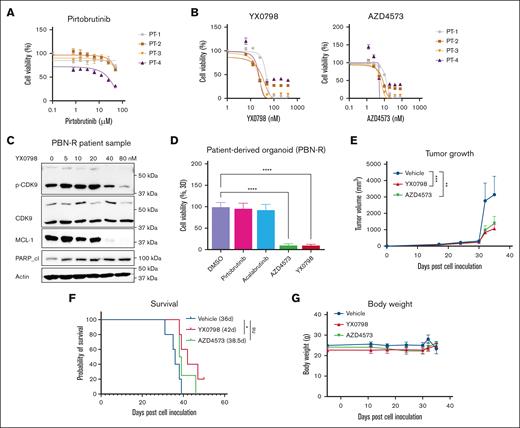
Orally administrated YX0798 effectively inhibits the growth of MCL cells from patients in cell culture and patient-derived models
To assess the anti-MCL efficacy of YX0798 within a clinical context, we first assessed its efficacy in primary MCL samples collected from patients with refractory/relapsed disease after treatment with BTKi (n = 4). As expected, the primary MCL cells no longer responded to BTK inhibition by pirtobrutinib (PBN) but were highly sensitive to CDK9 inhibition by YX0798 and AZD4573 (Figure 4A-B). As in MCL cell lines (Figure 2D), YX0798 induced dose-dependent inhibition of CDK9 phosphorylation, cleavage of PARP, and reduction of caspase-3 and MCL-1 (Figure 4C). To further address the efficacy of YX0798, we established PDO models from the same samples. These PDO models were again validated to be resistant to BTK inhibition. Importantly, AZD4573 and YX0798 were highly potent in inhibiting PDO growth (Figure 4D).
YX0798 is effective in inhibiting the tumor growth of PDX models in vivo. (A) Four primary samples originating from patients with BTKi-R/R MCL were confirmed to be resistant to BTKi PBN, (B) but were sensitive to CDK9is AZD4573 and YX0798. (C) YX0798 treatment for 6 hours inhibited CDK9 phosphorylation and induced cleavage of PARP, as well as MCL-1 depletion, in a dose-dependent manner in primary samples obtained from a patient with PBN-R. (D) CDK9is, such as AZD4573 or YX0798 (but not BTKis PBN or acalabrutinib), inhibited the growth of PDO models, which originated from the same patient samples used in panel C. (E-G) PDX models were established from a patient with sequential relapse to ibrutinib and CAR T-cell therapies. YX0798 was orally administered at 5 mg/kg (5 days on and 2 days off every week, equivalent to 25 mg/kg per week), whereas AZD4573 was intraperitoneally (IP) administered at 15 + 15 mg/kg (2 doses at 2 hours apart, once a week, equivalent to 30 mg/kg per week). (E) YX0798 treatment significantly inhibited tumor growth and (F) prolonged mouse survival. (G) Treatment with AZD4573 or YX0798 did not affect mouse body weight. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ns, not significant; p-CDK9, phosphorylated cyclin-dependent kinase 9; PBN, pirtobrutinib; PBN-R, pirtobrutinib resistance; PT, patient.
YX0798 is effective in inhibiting the tumor growth of PDX models in vivo. (A) Four primary samples originating from patients with BTKi-R/R MCL were confirmed to be resistant to BTKi PBN, (B) but were sensitive to CDK9is AZD4573 and YX0798. (C) YX0798 treatment for 6 hours inhibited CDK9 phosphorylation and induced cleavage of PARP, as well as MCL-1 depletion, in a dose-dependent manner in primary samples obtained from a patient with PBN-R. (D) CDK9is, such as AZD4573 or YX0798 (but not BTKis PBN or acalabrutinib), inhibited the growth of PDO models, which originated from the same patient samples used in panel C. (E-G) PDX models were established from a patient with sequential relapse to ibrutinib and CAR T-cell therapies. YX0798 was orally administered at 5 mg/kg (5 days on and 2 days off every week, equivalent to 25 mg/kg per week), whereas AZD4573 was intraperitoneally (IP) administered at 15 + 15 mg/kg (2 doses at 2 hours apart, once a week, equivalent to 30 mg/kg per week). (E) YX0798 treatment significantly inhibited tumor growth and (F) prolonged mouse survival. (G) Treatment with AZD4573 or YX0798 did not affect mouse body weight. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ns, not significant; p-CDK9, phosphorylated cyclin-dependent kinase 9; PBN, pirtobrutinib; PBN-R, pirtobrutinib resistance; PT, patient.
To further assess anti-MCL efficacy of YX0798, we generated a PDX model of relapsed MCL using a sample collected from a patient with sequential relapses to BTKi and CART-cell therapy right before the patient's decease. Currently, overcoming CAR T-cell therapy resistance is a major clinical challenge in treating MCL. In this PDX model, YX0798 was orally administrated at 5 mg/kg (5 days on and 2 days off every week, equivalent to 25 mg/kg per week), whereas AZD4573 was administrated interperitoneally at 15 + 15 mg/kg (2 doses at 2 hours apart, weekly, equivalent to 30 mg/kg per week). YX0798 not only suppressed tumor growth but also prolonged mouse survival for 7.4 days (Figure 4E-F), equivalent to 9.8 months of human life.25 Therefore, YX0798, but not AZD4573, showed significant treatment benefits by prolonging the survival of mice carrying this aggressive MCL xenograft (P < .05). No apparent side effects were seen during treatment with any of the dosing regimens (Figure 4G). To further assess YX0798 safety, we performed a toxicity test using a Chem 8 Panel (albumin, alanine aminotransferase, alkaline phosphatase, aspartate aminotransferase, blood urea nitrogen, creatinine, globulin, and total protein) for mouse plasma samples collected at the end of the experiment (Figure 4E-G). YX0798 did not cause significant alternations in any of the indicators (supplemental Figure 2). These data further support that oral administration of YX0798 at 5 mg/kg is safe and efficacious in this aggressive MCL model.
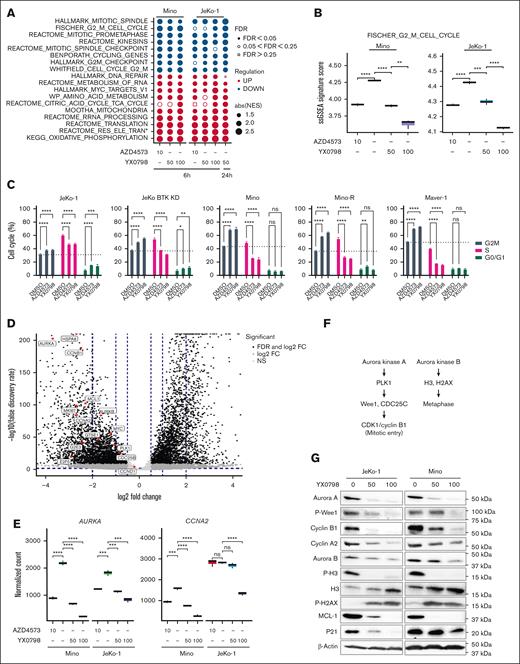
YX0798 triggers cell cycle arrest at G0/G1 phase
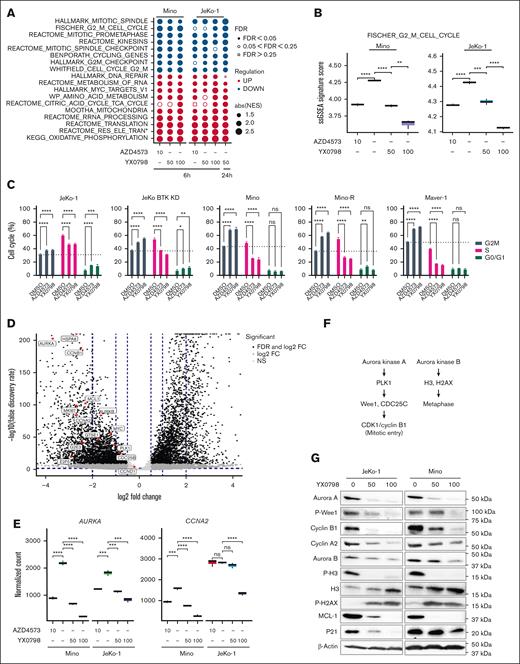
To understand the mechanism of action, we performed bulk RNA sequencing. Mino and JeKo-1 cells were treated with YX0798 or AZD4573 for 6 and 24 hours, respectively (supplemental Figure 3A). Consistent with the data in Figure 2C, YX0798 decreased cell viability in Mino and JeKo-1 cells in a dose-dependent manner as well as in a time-dependent manner (supplemental Figure 3B). At 6 hours after treatment, YX0798 and AZD4573 started to inhibit cell viability, which became more prominent at 24 hours (supplemental Figure 3B). For correlative studies, total RNA was isolated from aliquots of the same samples and subjected to bulk RNA sequencing. We sought to identify the effects of AZD4573 or YX0798 on transcriptomic changes. Gene set enrichment analysis (GSEA) revealed the signaling pathways that were significantly altered at 6 hours and further altered at 24 hours (Figure 5A). Multiple G2M-relevant and mitotic spindle-relevant pathways were the top downregulated pathways upon CDK9 inhibition, whereas cell metabolism-relevant pathways were the top upregulated pathways (Figure 5A). When JeKo-1 cells were treated with 50 nM YX0798 for 6 hours, multiple G2M- and mitosis-relevant signaling pathways started to become downregulated, which became significantly more downregulated when treated at 100 nM (false discovery rate of < 0.05; Figure 5A-B; supplemental Figure 3C). Similar data were also seen in Mino cells (Figure 5C; supplemental Figure 3C). These G2M signaling pathways were further downregulated when JeKo-1 cells were treated with 50 nM YX0798 for 24 hours (Figure 5A). Similarly, G2M pathways were downregulated upon AZD4573 treatment at 6 hours in both cell lines (Figure 5A; supplemental Figure 3C). This cumulative downregulation was rarely seen with other downregulated pathways (Figure 5A). Together, these data indicate that downregulation of G2M signaling pathways is a predominant transcriptomic change with CDK9 inhibition by either AZD4573 or YX0798 in MCL cells, in addition to depletion of c-MYC and MCL-1 (Figure 2D,F). Because they are the primary pathways downregulated by CDK9 inhibitors, they may drive the mechanism of action of CDK9i that leads to MCL cell death. For example, single-sample GSEA revealed that FISCHER_G2_M_CELL_CYCLE was significantly downregulated by CDK9is (Figure 5B). The inhibitors decreased the prevalence of cell cycle G2M pathways with enhanced effects when using higher doses or longer treatment durations (Figure 5B). These data led to our hypothesis that CDK9is disrupt cell cycle progression and lead to cell death. Indeed, treatment with either YX0798 or AZD4573 induced cell cycle arrest at the G0/G1 phase, not only in Mino and JeKo-1 cells but also in other more aggressive MCL cells, including BTKi-resistant JeKo BTK KD, Maver-1 cells, and Mino-R cells with acquired venetoclax resistance (Figure 5C).
YX0798 disrupts cell cycling in MCL cells toward cell cycle arrest. (A) The bubble plots show top cancer hallmarks and signaling pathways downregulated (blue) or upregulated (red) upon CDK9 inhibition. (B) The FISCHER_G2_M_CELL_CYCLE pathway was significantly downregulated by YX0798 (50 and 100 nM) in JeKo-1 cells (6 and 24 hours) and Mino cells (6 hours). AZD4573 (10 nM) serves as a control. (C) YX0798 and AZD4573 induced cell cycle arrest in MCL cells as indicated. (D) Volcano plot shows the significantly altered genes associated with cell cycle arrest upon YX0798 treatment at 100 nM for 6 hours. (E) Representative genes AURKA and CCNA2 were significantly downregulated with CDK9is YX0798 or AZD4573. (F) Illustration of Aurora kinase A– and B–mediated cell cycle progression. (G) Western blots confirmed cell cycle disruption induced by treatment with YX0798 and AZD4573 for 6 hours. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. FC, fold change; FDR, false discovery rate; absNES, absolute normalized enrichment score; ns, not significant; P-H2AX, phosphorylated histone 2A variant; P-H3, phosphorylated histone 3.
YX0798 disrupts cell cycling in MCL cells toward cell cycle arrest. (A) The bubble plots show top cancer hallmarks and signaling pathways downregulated (blue) or upregulated (red) upon CDK9 inhibition. (B) The FISCHER_G2_M_CELL_CYCLE pathway was significantly downregulated by YX0798 (50 and 100 nM) in JeKo-1 cells (6 and 24 hours) and Mino cells (6 hours). AZD4573 (10 nM) serves as a control. (C) YX0798 and AZD4573 induced cell cycle arrest in MCL cells as indicated. (D) Volcano plot shows the significantly altered genes associated with cell cycle arrest upon YX0798 treatment at 100 nM for 6 hours. (E) Representative genes AURKA and CCNA2 were significantly downregulated with CDK9is YX0798 or AZD4573. (F) Illustration of Aurora kinase A– and B–mediated cell cycle progression. (G) Western blots confirmed cell cycle disruption induced by treatment with YX0798 and AZD4573 for 6 hours. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. FC, fold change; FDR, false discovery rate; absNES, absolute normalized enrichment score; ns, not significant; P-H2AX, phosphorylated histone 2A variant; P-H3, phosphorylated histone 3.
To identify pivotal G2M genes responsible for cell cycle disruption, we performed differential gene expression analysis. Genes found to be significantly affected included HSP8A (heat shock protein family A [Hsp70] member 8), AURKA (Aurora Kinase A), CCNA2 (cyclin A2), CCNB1 (cyclin B1), CDC25B (cell sivision cycle 25B), PLK1 (polo-like kinase 1), E2F5 (E2F transcription factor 5), MKI67 (narker of proliferation Ki-67), G2E3 (G2M phase–specific E3 ubiquitin protein ligase), and GTSE1 (G2 and S-phase expressed 1; Figure 5D-E; supplementary Figure 3D-E). They were further validated by quantitative real-time polymerase chain reaction (supplemental Figure 4). With CDK9i inhibition, AURKA and CCNA2 mRNA decreased at 6 hours after treatment in Mino and JeKo-1 cells (Figure 5E). The AURKA-PLK1-Wee1-CDK1/CCNB1 axis is critical for mitotic entry and centrosome maturation, whereas AURKB-dependent histone H3 phosphorylation at Ser10 is a hallmark of mitosis26 (Figure 5F). Protein expressions of AURKA, CCNA2, CCNB1 and MCL-1 were indeed reduced with CDK9 inhibition at 6 hours, accompanied by reduced phosphorylation of Wee1 and histone H3 (Figure 5G). Together, these data suggested that Aurora kinases may be the primary players responsible for downregulated G2M pathways. Based on a library screen of 320 US Food and Drug Administration (FDA)–approved/investigational agents (SelleckChem, Houston, TX, USA), all 4 AURKA inhibitors in this library effectively inhibited the viability of all MCL cell lines tested (supplemental Figure 5A). Alisertib was especially effective in killing MCL cells (supplemental Figure 5B). These data support the idea that targeting Aurora kinases is an alternative approach for MCL treatment.
To further address the effect of CDK9i on cell cycle, we examined whether CDK9i causes cell cycle arrest earlier than apoptosis, or vice versa. At 6 hours after treatment, CDK9i induced cell cycle arrest at the G0/G1 phase (54.6% vs DMSO 47.5%) and started to induce apoptosis in only small fractions (11.9% vs DMSO 0.85%) of Mino cells. The remaining cells did not show any detectable caspase activity and were therefore deemed nonapoptotic (supplemental Figure 6A-B). Interestingly, cell cycle arrest at the G0/G1 phase (56.7% vs DMSO 47.2%) was readily observed in nonapoptotic cell fractions upon CDK9 inhibition (supplemental Figure 6B). Together, these data support that CDK9 inhibition causes cell cycle arrest before inducing apoptosis.
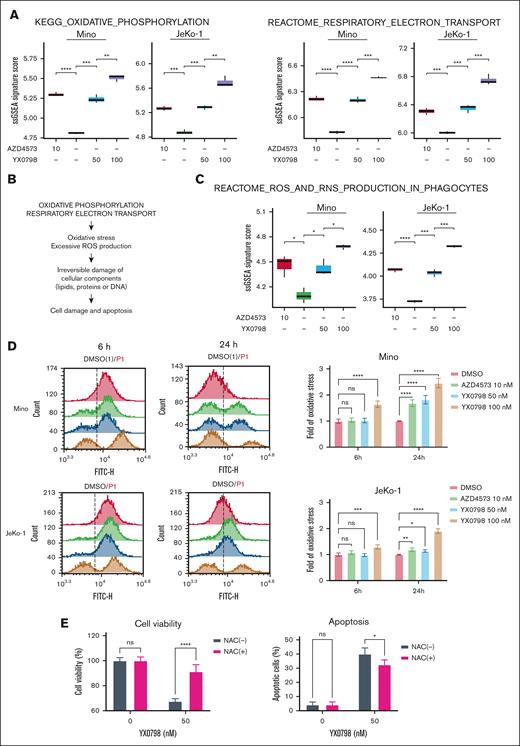
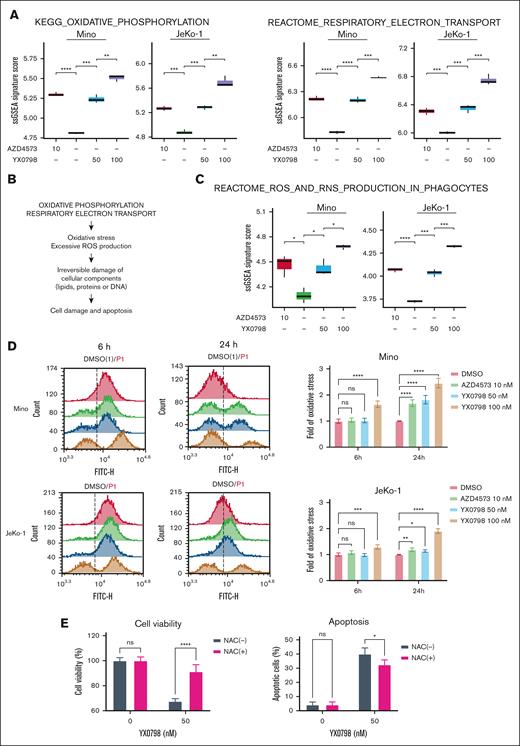
YX0798 induces ROS production
In addition to cell cycle disruption, CDK9i resulted in a remarkable enrichment of RESPIRATORY_ELECTRON_TRANSPORT and OXIDATIVE_PHOSPHORYLATION (OXPHOS) pathways (Figures 5A and 6A). Mitochondria are the main source of cellular reactie oxygen species (ROS), which is produced by the electron transport chain during oxidative phosphorylation.27 Therefore, it is likely that enrichment of these pathways leads to ROS overproduction, which irreversibly damages cellular components, including lipids, proteins, and DNA, leading to apoptosis28,29 (Figure 6B). Indeed, single-sample GSEA revealed that the REACTOME_ROS_AND_RNS_PRODUCTION_IN_PHAGOCYTES pathway was elevated by CDK9 inhibition (Figure 6C). Cellular ROS levels were elevated starting at 6 hours when JeKo-1 and Mino cells were treated with YX0798 at 100 nM, but not when treated at 50 nM or with AZD4573 at 10 nM. ROS elevation was detected at 6 and 24 hours after treatment in both cell lines in a dose- and time-dependent manner (Figure 6D; supplemental Figure 7). These findings correlate with reduced cell viability upon treatment (Figure 6D; supplemental Figure 3A). Furthermore, pretreatment with antioxidant N-acetyl cysteine (NAC) promoted cell survival and protected JeKo-1 cells from Y0798-triggered apoptosis at 5 hours after treatment (Figure 6E). Moreover, histone 2A variant X (H2AX) phosphorylation, an early indicator of DNA damage,30 was increased with YX0798 (Figure 5G). These data demonstrate that CDK9 inhibition in MCL cells leads to disrupted cell cycling and ROS production, which may orchestrate eventual cell death.
YX0798 induces enrichment of OXPHOS and excessive oxidative stress in MCL cells. (A) Enriched signaling pathways upon treatment with YX0798 and AZD4573 for 6 hours. (B) Illustration of enrichment of oxidative phosphorylation and RESPIRATORY_ELECTRON_TRANSPORT pathways leading to excessive oxidative stress and ROS production. (C) Enrichment of REACTOME_ROS_AND_RNS_PRODUCTION_IN_PHAGOCYTES signaling pathway upon treatment with YX0798 and AZD4573 for 6 hours. (D) ROS production is elevated upon treatment with YX0798 and AZD4573 for 6 and 24 hours in Mino and JeKo-1 cells. Flow cytometry results of cellular ROS levels in cells (left). Quantitative results of cellular ROS levels in cells (right). (E) NAC pretreatment for 16 hours promoted cell survival and protected JeKo-1 cells from apoptosis induced by YX0798 treatment for 5 hours. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. NAC, N-acetyl cysteine; ns, not significant; ssGSEA, single-sample GSEA.
YX0798 induces enrichment of OXPHOS and excessive oxidative stress in MCL cells. (A) Enriched signaling pathways upon treatment with YX0798 and AZD4573 for 6 hours. (B) Illustration of enrichment of oxidative phosphorylation and RESPIRATORY_ELECTRON_TRANSPORT pathways leading to excessive oxidative stress and ROS production. (C) Enrichment of REACTOME_ROS_AND_RNS_PRODUCTION_IN_PHAGOCYTES signaling pathway upon treatment with YX0798 and AZD4573 for 6 hours. (D) ROS production is elevated upon treatment with YX0798 and AZD4573 for 6 and 24 hours in Mino and JeKo-1 cells. Flow cytometry results of cellular ROS levels in cells (left). Quantitative results of cellular ROS levels in cells (right). (E) NAC pretreatment for 16 hours promoted cell survival and protected JeKo-1 cells from apoptosis induced by YX0798 treatment for 5 hours. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. NAC, N-acetyl cysteine; ns, not significant; ssGSEA, single-sample GSEA.
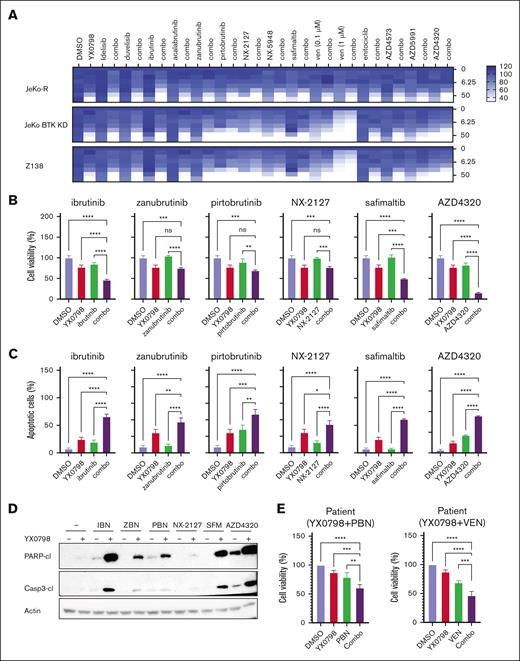
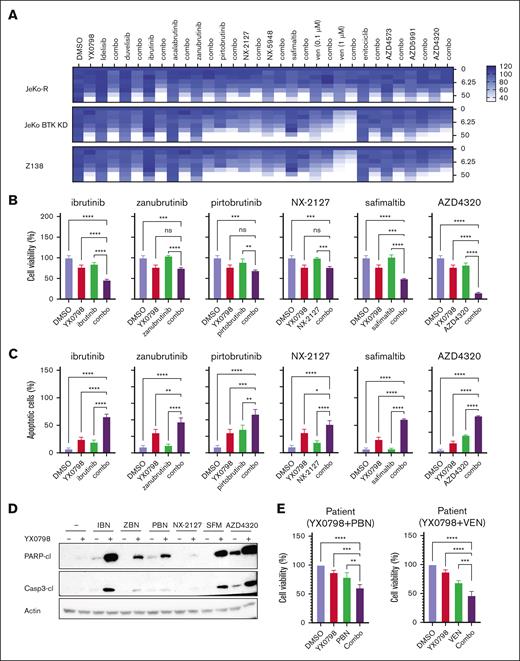
YX0798 shows potential for combined treatment with clinical agents
To improve treatment efficacy, we evaluated the potential of YX0798 in overcoming therapeutic resistance by combining other agents that are FDA approved or under clinical investigation in BTKi-resistant cells. BTKis (ibrutinib, zanubrutinib and pirtobrutinib) and a degrader (NX-2127), a mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT1) inhibitor (safimaltib), and an MCL-1 inhibitor (AZD4320) showed good combinational effect (Figure 7A). YX0798 greatly enhanced the anti-MCL potency of these agents (Figure 7B-C; supplemental Figure 8A-B). YX0798 dramatically enhanced cleavage of PARP and caspase-3 in JeKo-1 cells. In addition, YX0798 augmented the ex vivo efficacy of pirtobrutinib and venetoclax in a pirtobrutinib-resistant primary patient sample (Figure 7E). These data demonstrate that YX0798 has great potential to be used in combination therapy to improve treatment efficacy.
YX0798 shows the potential for combined treatment with other agents. (A) In JeKo-1, JeKo BTK KD, and Z138 cells, a combinational drug screen for YX0798 with a list of agents that are FDA approved or under clinical investigation. The y-axis on the right shows the concentration of YX0798 (nanomolar). The color coding for drug efficacy is shown in the legend. (B) In JeKo-1 cells, YX0798 decreased cell viability and (C) increased apoptosis when used in combination with ibrutinib, zanubrutinib, pirtobrutinib, NX-2127, safimaltib, or AZD4320. (D) Western blots show YX0798 enhanced the cleavage of PARP and caspase-3 when combined with ibrutinib, zanubrutinib, pirtobrutinib, NX-2127, safimaltib, or AZD4320. (E) YX0798 decreased cell viability when used in combination with PBN or VEN in primary samples obtained from a PBN-resistant patient. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ns, not significant; VEN, venetoclax.
YX0798 shows the potential for combined treatment with other agents. (A) In JeKo-1, JeKo BTK KD, and Z138 cells, a combinational drug screen for YX0798 with a list of agents that are FDA approved or under clinical investigation. The y-axis on the right shows the concentration of YX0798 (nanomolar). The color coding for drug efficacy is shown in the legend. (B) In JeKo-1 cells, YX0798 decreased cell viability and (C) increased apoptosis when used in combination with ibrutinib, zanubrutinib, pirtobrutinib, NX-2127, safimaltib, or AZD4320. (D) Western blots show YX0798 enhanced the cleavage of PARP and caspase-3 when combined with ibrutinib, zanubrutinib, pirtobrutinib, NX-2127, safimaltib, or AZD4320. (E) YX0798 decreased cell viability when used in combination with PBN or VEN in primary samples obtained from a PBN-resistant patient. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ns, not significant; VEN, venetoclax.
Discussion
Our study demonstrated that YX0798 is a highly potent and selective CDK9i with oral bioavailability that not only depletes the expression of oncogenic c-MYC and prosurvival MCL-1 but also drives transcriptomic reprogramming toward cell cycle dysregulation and ROS production.
CDK9 has long been recognized as a valuable target for therapeutic development in treating cancer.10 Early compounds that targeted CDK9 were essentially pan-CDKis, targeting multiple CDKs. For example, flavopiridol targets CDK1, CDK2, CDK4, and CDK6, and less potently CDK9,31,32 whereas seliciclib targets CDK1, CDK2, and CDK5 and less potently CDK9.33 Pan-CDKis show suboptimal clinical benefits with adverse effects, leading to failed clinical trials.10 Therefore, it is critical to develop exquisite CDK9-specific inhibitors. Recently generated CDK9is, such as AZD457324 and enitociclib,17 demonstrate high CDK9 selectivity and are currently under clinical investigation; however, they can only be administered by IV infusion, and early clinical data showed limited treatment responses accompanied by toxicity. Here, we aimed to develop a potent and selective CDK9i with acceptable oral bioavailability. Compared to AZD4573, YX0798 showed higher target selectivity. Furthermore, YX0798 demonstrated oral bioavailability and was efficacious in overcoming therapeutic resistance. These results show the advantages of YX0798 over other CDK9is, such as AZD4573 and enitociclib, which have no reported oral bioavailability.
For AZD4573 and enitociclib, depletion of c-MYC and MCL-1 is the primary mechanism of action of CDK9 inhibition.17,18 This is also the case for YX0798. Unexpectedly, bulk RNA sequencing showed that HALLMARK_MYC_TARGETS_V1 was enriched upon treatment for 6 hours. Studies using other CDK9is, such as i-CDK9 and flavopiridol, reported a transient c-MYC increase in other cancer cells due to a BRD4-dependent compensatory pathway.34 Therefore, our data confirm that transient c-MYC increase is a common mechanism across CDK9is, which may explain YX0798-triggered enrichment of the HALLMARK_MYC_TARGETS_V1 pathway upon 6 hours of treatment. In addition, YX0798 treatment not only blocks CDK9 phosphorylation, but also reduces CDK9 total protein expression, albeit to a much lesser extent. CDK9 protein has a half-life of 4 to 7 hours.35 It is likely that CDK9 inhibition results in blocking CDK9 inhibition, which in turn switches off CDK9 transcription and translation, leading to reduced protein expression. The exact mechanism requires further investigation.
Our transcriptomic profiling discovered that YX0798 and AZD4573 resulted in remarkable downregulation of G2M signaling pathways, which likely leads to cell cycle arrest at the G0/G1 phase and has not been reported for earlier generations of CDK9-specific inhibitors. Therefore, this is the first time that cell cycle disruption has been revealed as a primary mechanism of CDK9-specific inhibition. Transcriptomic profiling also revealed that CDK9is trigger enrichment of OXPHOS and cellular ROS production in MCL cells. AZD4573 was reported to upregulate OXPHOS cancer hallmarks in MCL mouse models.25 Consistent with that, both YX0798 and AZD4573 result in enrichment of OXPHOS and mitochondrial RESPIRATORY_ELECTRON_TRANSPORT pathways in MCL cell lines. ROS is predominantly produced by mitochondria, originating from the electron transport chain during the OXPHOS process.27 Therefore, it is not surprising that CDK9 inhibition results in enrichment of REACTOME_ROS_AND_RNS_PRODUCTION_IN_PHAGOCYTES. Together, our findings may explain why we detected excessive cellular ROS upon CDK9 inhibition. Overproduction of ROS leads to damage of cellular components, including DNA,28,29 as evident by H2AX phosphorylation.
Our previous study reports that OXPHOS is highly associated with ibrutinib resistance in MCL and demonstrated its important role in contributing to ibrutinib resistance.12,14 CDK9 inhibition by enitociclib36 and AZD4573 has the potential to overcome therapeutic resistance in MCL. Consistent with this, YX0798 can also overcome therapeutic resistance, including resistance to BTKis and CAR T-cell therapy. Furthermore, YX0798 enhanced the in vitro anti-MCL activity when combined with other agents that are FDA approved or under clinical investigation, including BTKis (ibrutinib, zanubrutinib, and pirtobrutinib) and degrader (NX-2127), MALT1 inhibitor (safimaltib), and MCL-1 inhibitor (AZD4320). These data demonstrate that YX0798 has great potential to be used in combination therapy with clinical agents to improve treatment efficacy and patient outcome.
Acknowledgments
The authors thank the patients and their families who contributed to this research study. The authors also thank Tracey Baas for technical editing.
This work is supported by a grant from the Cancer Prevention and Research Institute of Texas (RP210062 [M.W. and J.Z.]), an Institutional Research Grant award from MD Anderson Cancer Center (V.J.), Cancer Center Support Grant for MD Anderson Cancer Center from National Cancer Institute, the Steve and Nancy Fox Cancer Research Fund (M.W.), the John D. Stobo, M.D. Distinguished Chair Endowment (J.Z.), and the Edith & Robert Zinn Chair Endowment in Drug Discovery (J.Z.).
Authorship
Contribution: M.W., J.Z., and V.J. conceived the idea; V.J., J.Z., and Y.X. designed the experiments; V.J., Y.X., H.K., T.Z., L.N., J.M., Y.L., and H.C. performed the experiments; V.J., Y.X., H.K., and Q.C. performed data analysis; V.J. and Y.X. directed and coordinated the project and wrote the manuscript; V.J., Y.X., J.Z., and M.W. contributed to the manuscript revision; M.W., J.Z., and V.J. acquired the funding; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: M.W. reports consulting for Acerta Pharma, AstraZeneca, Bristol Myers Squibb, Boxer Capital, Galapagos NV, Genmab, InnoCare, Janssen, Kite Pharma, Lilly, Merck, Physicians Education Resources (PER), Pfizer, and Oncternal; receiving research funding from AbbVie, Acerta Pharma, AstraZeneca, Bantam Pharma, BeiGene, BioInvent, Celgene, Genmab, Genentech, Innocare, Janssen, Juno Therapeutics, Kite Pharma, Lilly, Loxo Oncology, Molecular Templates, Nurix Therapeutics, Oncternal, Pharmacyclics, VelosBio, and Vincerx; and receiving honoraria from AstraZeneca, BeiGene, Binaytara Foundation, Bristol Myers Squibb, Chinese American Hematologist and Oncologist Network (CAHON), Editorial Medica AWWE SA, East Virtinia Medical School, Istituto Scientifico Romagnolo, Janssen, Kite Pharma, Mayo Clinic, MJH Life Sciences, Merck, Maria Sklodowska-Curie (MSC) National Research Institute of Oncology, Pfizer, PER, Plexus Communications, PromCon S.R.E., Research To Practice, Studio ER Congressi, South African Clinical Hematology Society, Medscape/WebMD, and VJHemOnc. The remaining authors declare no competing financial interests.
Correspondence: Michael Wang, Department of Lymphoma and Myeloma, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; email: miwang@mdanderson.org; Jia Zhou, Department of Pharmacology and Toxicology, The University of Texas Medical Branch, 301 University Blvd, Galveston, TX 77555; email: jizhou@UTMB.edu; and Vivian Jiang, Department of Lymphoma and Myeloma, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; email: vjiang@mdanderson.org.
References
Author notes
V.J. and Y.X contributed equally to this study.
The data set is available in the European Genome-phenome Archive (accession number EGAD50000001582).
Original data are available on request from the corresponding author, Vivian Jang (vjiang@mdanderson.org).
The full-text version of this article contains a data supplement.