Anaplastic large cell lymphoma (ALCL) is a rare form of mature T-cell non-Hodgkin lymphoma. In pediatric patients, most cases are anaplastic lymphoma kinase (ALK) positive. Despite intensive multiagent chemotherapy regimens, treatment failure rates remain at 25% to 30%. Recent advancements in targeted therapies, notably ALK inhibitors and the anti-CD30 antibody-drug conjugate brentuximab vedotin have demonstrated substantial activity in relapsed and refractory settings. Molecular detection of minimal disseminated disease (MDD) and minimal residual disease (MRD) offers improved prognostic stratification. For patients with relapsed or refractory disease, targeted therapies have increased treatment options, but more work needs to be done to define optimal treatment regimens, duration, and need for hematopoietic stem cell transplantation in this group. Immune therapies such as checkpoint inhibitors or chimeric antigen receptor T-cell therapy provide additional therapeutic options. Incorporating targeted therapies and MDD/MRD assessments into clinical trials could significantly improve outcomes for pediatric and adolescent patients with ALCL.
Introduction
Anaplastic large cell lymphoma (ALCL) is an aggressive T-cell lymphoma characterized by large, pleomorphic cells expressing CD30, first described in 1985.1 The World Health Organization now recognizes 2 entities, anaplastic lymphoma kinase–positive (ALK+) and ALK− ALCL.2 Constitutively expressed ALK is the driver of tumorigenesis in ALK+ ALCL, most frequently with the t(2;5)(p23;q35) translocation, which fuses the nucleolar phosphoprotein gene nucleophosmin 1 (NPM) to ALK.3 ALCL represents 10% to 15% of pediatric and adolescent non-Hodgkin lymphoma (NHL), and nearly 95% of cases in this age group are ALK+.4 A wide range of chemotherapy strategies has been trialed to improve frontline treatment of patients with ALCL; however, no regimen has significantly reduced the failure rate of 25% to 30%.5-17
The development of targeted therapies has created an opportunity for a paradigm shift in the approach to ALCL treatment. Because the vast majority of pediatric ALCL cases are ALK+, they are prime targets for drugs that inhibit ALK phosphorylation, resulting in antitumor activity. ALCL also uniformly expresses CD30 on the cell surface, which can be targeted by brentuximab vedotin (BV), a CD30-targeted antibody conjugated to monomethyl auristatin E. Advances in measuring circulating tumor cells in peripheral blood and bone marrow using polymerase chain reaction (PCR) to target the ALK-associated fusion gene to detect minimal disseminated disease (MDD) at diagnosis and minimal residual disease (MRD) present opportunities for improved risk stratification and prognostication.18-20 Although these developments have primarily been studied in relapsed or refractory (R/R) settings, both targeted therapies and MDD/MRD assessment have the potential to be incorporated into up-front prospective trials and improve therapy for all patients with pediatric ALCL.
Clinical case
A 12-year-old boy presents with 1 week of fever, cervical lymphadenopathy, and abdominal pain. Physical examination reveals bilateral firm, nontender, enlarged lymph nodes and splenomegaly. Significant laboratory findings include anemia, thrombocytopenia, hypertriglyceridemia, and hyperferritinemia. After cervical lymph node and bone marrow biopsy, dexamethasone is initiated due to concern for worsening clinical status and hemophagocytic lymphohistiocytosis (HLH). Lymph node biopsy shows large CD30+ cells with abnormal horseshoe-shaped nuclei and strong ALK staining, confirming ALCL. Bone marrow shows hemophagocytosis but no blasts. Imaging shows disseminated disease in lymph nodes above and below the diaphragm. Central nervous system (CNS) disease is not present. He is diagnosed with stage III disease and started on treatment as per Children’s Oncology Group (COG) ANHL12P1 with BV, with a prolonged dexamethasone taper to help control inflammation from HLH.
Clinical presentation and diagnosis
ALCL often presents with advanced stage disease, extranodal involvement, and systemic symptoms such as fever and weight loss.21 Atypical presentations with fluctuating lymph node enlargement or other variable symptoms may occasionally occur. In ∼10% of cases, ALCL is complicated by HLH, although outcomes are similar to those without HLH.22
ALCL diagnosis requires tissue biopsy, with excisional or core biopsies preferred over fine-needle aspiration, which often provides insufficient material for complete analysis. Staging includes physical examination, computed tomography scans or magnetic resonance imaging, positron emission tomography if feasible, and bone marrow and cerebrospinal fluid assessments. Historically, patients were classified using the Murphy/St Jude Staging system according to involved anatomic sites and the extent of bone marrow and/or CNS involvement, which has now been updated to the revised International Pediatric NHL Scoring System.23,24
Frontline treatment for pediatric ALCL
Before the recognition of ALCL as a distinct form of NHL, most patients were treated as having B- or T-cell NHL with diverse regimens. Due to the limited number of pediatric patients with ALCL, there have been few randomized trials. Moreover, the series of trials performed to date resulted in similar event-free survival (EFS) rates of 65% to 75% across different strategies (Table 1). The NHL–Berlin-Frankfurt-Münster (NHL-BFM) working group trial NHL-BFM 90 was, to our knowledge, the first to specifically include an arm for ALCL, building on previous B-cell NHL regimens with multiagent chemotherapy.7
The European Intergroup for Childhood NHL (EICNHL) ran ALCL99, the first international randomized trial for patients with ALCL aged <22 years, using chemotherapy based on NHL-BFM 90 and demonstrated a 2-year EFS of 74% and overall survival (OS) of 92.5% and a 10-year EFS of 70% and OS of 90%.12,27 Stage I patients with completely resected disease received short-course chemotherapy with a 2-year EFS of 100%, whereas those with incompletely resected disease received standard therapy and had outcomes similar to those of higher-stage patients.28 The trial also demonstrated that intermediate-dose methotrexate (3 g/m2) with shorter infusion duration (>3 hours) and no intrathecal therapy provided the same EFS as other methotrexate schedules, with less toxicity.12 Vinblastine maintenance was evaluated as part of this trial, based on previous data showing its efficacy in relapsed ALCL. Although patients who received vinblastine maintenance therapy to complete 1 year of total treatment had a significantly reduced risk of events during the first year (1-year EFS, 91% vs 74% without vinblastine), this benefit did not persist, and there was no difference in 2-year EFS (73% vs 70% without vinblastine).13
The COG trial ANHL0131 investigated the addition of vinblastine in place of vincristine in standard APO (doxorubicin [Adriamycin], prednisone, and vincristine [Oncovin]) treatment, which included 300 mg/m2 of anthracycline but no alkylating agents. No difference in survival was seen between the 2 arms (3-year EFS, 74% with vincristine vs 79% with vinblastine), and additional toxicity was noted with vinblastine.14
The goal of the most recent COG trial ANHL12P1 was to test the addition of targeted therapy to the standard ALCL99 chemotherapy backbone. This approach aimed to use a lower cumulative anthracycline dose and shorter duration of therapy, while incorporating either crizotinib (an ALK inhibitor) or BV.29-31 Patients in the crizotinib arm of the study had a 2-year EFS of 77% and OS of 95%, whereas patients in the BV arm of the study had a 2-year EFS of 79% and OS of 97%.15,16 BV did not cause additional toxicity; however, the addition of crizotinib to chemotherapy led to an increase in thromboembolic events.
A trial from the Pediatric Lymphoma Collaboration Group in Southern China, SCCCG-ALCL-2017 (ClinicalTrials.gov identifier: NCT03971305), used a similar chemotherapy backbone and intensified chemotherapy for patients who were considered high risk (MDD positive, high-risk site, or small cell/lymphohistiocytic variant).25 Patients with suspected residual disease by imaging at the end of therapy were nonrandomly assigned to receive a year of crizotinib (7 patients; no relapses) or vinblastine (5 patients; 1 relapse). Three-year EFS was 90% for the intermediate-risk group and 68% for the high-risk group, suggesting that intensification of chemotherapy alone will not overcome poor outcomes in high-risk patients.
Frontline treatment for young adult ALCL
Young adults have a higher frequency of ALK− ALCL than children, although they are more likely to have ALK+ ALCL than older adults.32 Standard adult treatment for ALCL most often uses CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or other anthracycline-containing chemotherapy backbones for aggressive lymphomas, with better outcomes noted for ALK+ than ALK− cases.32-37 The addition of etoposide to CHOP (CHEOP) was found to be beneficial for young patients (aged <60 years) with ALK+ ALCL and normal lactate dehydrogenase, with a 3-year EFS 91.2% vs 57.1% for those receiving CHOP.36 Similar to pediatrics, the limited number of adults with ALCL makes randomized controlled trials challenging, and most trials combine patients with ALCL with those with other peripheral T-cell lymphomas.
Building off of a promising phase 1 trial in adults incorporating BV with cyclophosphamide, doxorubicin, and prednisone (BV+CHP) in the treatment of CD30-expressing peripheral T-cell lymphomas,38 the phase 3 ECHELON-2 trial demonstrated the safety and efficacy of BV+CHP in adults with newly diagnosed ALCL (including ALK− ALCL and ALK+ ALCL with International Prognostic Score of ≥2), with an estimated 5-year progression-free survival (PFS) of 60.6% and OS of 75.8%.26,39 ECHELON-2 set a new standard for the treatment of adult patients with ALK+ ALCL. From a young adult perspective, comparing treatment strategies, outcomes with BV+CHP approach those seen with the more intensive ALCL99/ANHL12P1 backbone. However, the high anthracycline dose (300-400 mg/m2) of BV+CHP is concerning due to the risk of long-term cardiac toxicity, whereas ALCL99/ANHL12P1 limits anthracycline exposure to 150 mg/m2. Another trial incorporating etoposide into the BV+CHP regimen followed by BV consolidation is currently underway with preliminary results showing safety and efficacy. However, the study is primarily focused on peripheral T-cell lymphomas other than ALCL due to the unmet need in those diagnoses and the promising results of ECHELON-2.40 This study will be informative to better understand the potential role of BV consolidation in the up-front treatment of ALCL, which could be translated into pediatric treatment approaches.
Current frontline clinical trial
The current EICNHL trial for pediatric patients with newly diagnosed ALK+ ALCL tests the efficacy of 2 years of weekly vinblastine monotherapy for children with stage I to III disease who are MDD negative (www.clinicaltrialsregister.eu identifier: EudraCT 2017-002935-40).
Prognostic factors
Understanding prognostic factors in ALCL is critical for developing risk-adapted treatment strategies. Although several trials have identified clinical risk factors such as B-symptoms, bone marrow involvement, or involvement of the lungs, liver, spleen, or skin, none have been consistently prognostic across trials.7,11,41 Small cell and lymphohistiocytic variants are associated with worse prognosis, but their utility is complicated by interobserver variability.27,42 Autoantibody response to ALK at the time of diagnosis has been inversely correlated with lymphoma dissemination and relapse risk in ALK+ ALCL and could potentially serve as a prognostic marker.43 Finally, recent genomic analysis suggests that there is molecular heterogeneity within pediatric ALK+ ALCL, which could provide insight into disease pathogenesis and provide additional prognostic markers.44,45
MDD and MRD
MDD and MRD, analyzed by qualitative real-time PCR for NPM::ALK fusion transcripts, are now validated prognostic factors in pediatric ALK+ ALCL.18,19 MDD positivity is associated with a higher cumulative incidence of relapse (50% vs 15%), lower 5-year EFS (38% vs 82%), and lower OS (60% vs 86%) than those with negative MDD.18 MDD from peripheral blood at diagnosis was also shown to be predictive of outcome in the ANHL12P1 study.15,16
Digital PCR has recently emerged as a promising method for MDD detection and quantification due to its higher reproducibility across laboratories. Using a previously described cutoff of 30 normalized copy numbers (NCNs) in bone marrow and/or peripheral blood, patients treated with ALCL99 chemotherapy with ≥30 NCNs at diagnosis were found to have a significantly lower 5-year PFS of 34% than those with <30 NCNs (74%) or those testing negative (76%).46 The persistence of MRD after the first cycle of chemotherapy using qualitative real-time PCR further identifies a very high–risk group of MDD+/MRD+ patients, with a cumulative incidence of relapse of 81%, significantly higher than that of MDD+/MRD− patients (31%) and MDD− patients (15%).46
Despite strong evidence of the prognostic significance of MDD and its use for risk stratification in Europe, MDD assessment has not been routinely used in clinical practice in North America due to the absence of a Clinical Laboratory Improvement Amendments–approved test. Work is underway to make this important test available for all patients with ALK+ ALCL.
Recommendation
Based on previous trials, we recommend treating patients with stage II to IV ALK+ ALCL as per the ALCL99/ANHL12P1 chemotherapy backbone, with the addition of BV when available.
Clinical case continued
A 12-year-old boy with stage III ALK-positive ALCL completed therapy with ANHL12P1+BV. Three months later, he presents to clinic with new cervical lymphadenopathy and fatigue. Positron emission tomography scan shows F-18 fluorodeoxyglucoose avidity in cervical, mediastinal, and mesenteric lymph nodes. Cervical lymph node biopsy confirms relapsed ALK+ ALCL, and cerebrospinal fluid and bone marrow evaluations are negative. He is started on treatment with a dexamethasone pulse and crizotinib. After 1 month of crizotinib treatment, imaging shows complete response (CR). He receives another month of crizotinib, followed by matched 10/10 sibling allogeneic hematopoietic stem cell transplant (allo-HSCT) with a reduced-intensity conditioning regimen consisting of fludarabine, alemtuzumab, and 600 cGy of total body irradiation. Unfortunately, 3 months after HSCT, he relapses again with cervical and mesenteric lymphadenopathy. He restarts crizotinib and achieves a CR after 2 months of therapy. He continues in remission for 2 years while taking crizotinib, and the family wants to discuss with his physician when it would be safe to stop crizotinib.
Management of R/R ALK+ ALCL
A range of treatment strategies from vinblastine monotherapy, ALK inhibitors, and BV to allo-HSCT have been used to treat R/R ALK+ ALCL. Time from diagnosis to relapse is an independent risk factor for survival, with patients who progressed on initial therapy having the worst prognosis.47-49 The EICNHL developed the prospective ALCL-Relapse trial, which ran from 2004 to 2014, to test a risk-stratified approach for treating pediatric patients with R/R ALCL, building on a previous retrospective study.47,50 Low-risk patients (CD3– and relapse >12 months from diagnosis) received weekly vinblastine monotherapy for 24 months, achieving a 5-year EFS of 81% and OS of 90%. For intermediate-risk patients (CD3– and relapse within 1 year) treated with chemotherapy followed by autologous HSCT (auto-HSCT), the study found that auto-HSCT was ineffective, with 70% of patients experiencing relapse, progression, or death. Very high–risk patients (progressive disease during initial therapy) and high-risk patients (CD3+ relapse) were treated with chemotherapy and allo-HSCT, with 5-year EFS rates of 41% and 62% and OS 59% and 73%, respectively. Notably, 50% of very high–risk patients progressed on reinduction chemotherapy before transplant, highlighting the need for alternative treatment approaches. Although this study established the standard of consolidation with allo-HSCT for pediatric patients with R/R ALK+ ALCL with progressive disease or early relapse, treatment options have evolved since it was completed (Table 2). Future trials for R/R ALCL must evaluate how to incorporate targeted therapies for reinduction before HSCT and further define which patients may not need HSCT for cure.
ALK inhibitors
ALK inhibitors were developed to prevent the proliferation of cancer cells driven by continuous ALK activation. These drugs were initially developed primarily for ALK+ non–small cell lung cancer but also have clinical potential for treating ALCL.57 Crizotinib, a first-generation ALK inhibitor, was approved by the US Food and Drug Administration in 2021 for the treatment of relapsed ALK+ ALCL based on the COG study, ADVL0912 (NCT00939770). In this study, 26 patients with relapsed ALK+ ALCL were treated with crizotinib and achieved an objective response rate of 88%.29 The French phase 2 AcSé-crizotinib trial also evaluated crizotinib use in patients with R/R ALK+ ALCL, demonstrating a response rate at 8 weeks of 67%, with a 3-year PFS of 40% and OS of 63%.53 Crizotinib also demonstrated good efficacy in adult trials of ALK+ lymphomas (NCT02419287 and EudraCT 2010-022978-14) at a single institution, with a 2-year PFS of 57% and OS of 58%.54 In all studies, crizotinib was well tolerated, with occasional neutropenia, visual disturbances, and gastrointestinal events.
Next-generation ALK inhibitors, including alectinib, ceritinib, brigatinib, and lorlatinib, are more potent than crizotinib and have the benefit of CNS penetration. A trial of alectinib in patients with R/R ALK+ ALCL had an overall response rate (ORR) of 80% and led to the approval of alectinib for this population.51 A trial of ceritinib (NCT01742286) had an ORR of 75% in 12 patients with R/R ALK+ ALCL.52 Brigatinib is currently being studied in the BrigaPED trial, a phase 1/2 trial for adults and children with R/R ALK+ ALCL (NCT04925609), with preliminary results demonstrating safety and efficacy.56 The activity of the third-generation ALK inhibitor lorlatinib has not yet been explored in children with ALK+ ALCL, but a trial including adult patients previously treated with other ALK inhibitors is open for accrual in Italy (NCT03505554). No trial to date has compared the efficacy of different ALK inhibitors in ALCL.
Significant questions remain regarding whether treatment with ALK inhibitors can be curative in R/R ALK+ ALCL and whether there is an optimal duration of treatment. Although many patients respond to ALK inhibitors, rapid relapse after discontinuation of crizotinib has been reported.58 The relapse studies described above have a subset of patients who continued on ALK inhibitors without HSCT or other therapy, but there are no prospective trials monitoring outcomes in these patients. Consequently, in the absence of evidence, many patients continue ALK inhibitors for many years. In a retrospective, real-world study of pediatric and adolescent patients with R/R ALK+ ALCL, 37 of 81 (46%) received ALK inhibitor monotherapy as their initial reinduction regimen, with 92% of those patients achieving a CR.59 Seventy percent of patients proceeded to HSCT after ALK inhibitor reinduction, and 30% continued without HSCT. Of the 11 patients who did not undergo transplant, 5 discontinued ALK inhibitor therapy. Of those, 1 relapsed but achieved a second CR after restarting the same ALK inhibitor, and 4 remain in remission off therapy. Although this is suggestive that ALK inhibitor therapy may be sufficient treatment for some patients, there are still unanswered questions about which patients (potentially those with low-stage disease or late relapse) should be considered for this treatment option and how long they should receive treatment. A better understanding of the long-term side effects of ALK inhibitors is also critical to help guide these decisions.
BV
Another targeted therapy with proven efficacy in R/R ALCL is BV. Initial data on BV monotherapy in ALCL came from its use in patients with R/R ALCL, with CR rates of 41% to 66%.30,31 In a study of adults with R/R ALCL, 50 of 58 patients (86%) achieved an objective response with BV monotherapy. Although most patients proceeded to HSCT, 8 of the 38 patients (21%) who achieved a CR maintained remission without additional therapy. In a study of BV monotherapy in 17 pediatric patients with R/R ALCL, 53% achieved an overall response (NCT01492088).31 Peripheral neuropathy is the main side effect reported and is more common in adults.30,31,39 As BV is more frequently incorporated into up-front therapy, it is unclear whether it will maintain as much efficacy in the relapse setting; however, a study on re-treatment with BV in ALCL found an ORR of 88% in patients with ALCL.60
Vinblastine
Although weekly vinblastine for 2 years is generally considered the standard of care for pediatric patients with a late relapse of ALCL in Europe,50,61 it has not been widely adopted in the United States. Building on prior evidence of efficacy of vinblastine in R/R ALCL,62 the ALCL-Relapse trial treated low-risk patients with weekly vinblastine monotherapy for 24 months, achieving a 5-year EFS of 81%.50 Despite this efficacy, the retrospective, real-world trial from the United States showed that only 5% of R/R patients with ALK+ ALCL received vinblastine as their first-line reinduction regimen, even though 35% of patients relapsed >1 year after initial diagnosis.59 The reasons behind these different treatment approaches are multifactorial but may be related to the logistical challenges of weekly hospital visits for 2 years, the availability of newer targeted therapies, and the toxicity of weekly vinblastine.
The CRISP trial (EudraCT 2015-005437-53) was designed to study the combination of vinblastine and crizotinib in high-risk R/R ALK+ ALCL.55 Unfortunately, the combination was found to be too toxic, resulting in significant febrile neutropenia and gastrointestinal events, with the study reaching stopping rules before accrual. For the 13 patients who received the combination treatment, efficacy was good for this high-risk population, with a 2-year EFS of 77% and OS of 85%. Additional prospective trials in R/R ALCL are needed to find optimal therapeutic combinations.
Checkpoint inhibitors
Immune checkpoint inhibition is another potential treatment approach, because programmed death ligand 1 is expressed in ALCL and immune response plays an essential role in disease control.63 Case reports have demonstrated good responses to the programmed death ligand 1 inhibitors nivolumab and pembrolizumab in the treatment of patients with R/R ALCL.64-66 Nivolumab monotherapy is currently being studied in Europe in the NIVOALCL trial (NCT03703050) in pediatric and adult patients with R/R ALCL with progression after targeted therapy or as consolidative immunotherapy.
CAR-T therapy
Chimeric antigen receptor T-cell (CAR-T) therapy targeting CD30 has been developed primarily for patients with R/R Hodgkin lymphoma but also shows preliminary efficacy in patients with ALCL.67-70 In a trial (NCT01316146) of 2 patients infused with anti-CD30 CAR-Ts without a conditioning regimen, 1 achieved a CR that persisted for 9 months.67 In another trial with lymphodepletion before anti-CD30 CAR-T infusion (ChiCTR-OPN16009069), 2 of 3 patients with ALCL achieved a prolonged partial response, whereas 1 had progressive disease.68 Further trials are needed to optimize CAR-T therapy in this rare group of patients.
To transplant or not to transplant
There is a lack of evidence to guide decisions regarding which patients would benefit the most from a transplant in an era of expanding treatment options. In pediatric patients, the ALCL-Relapse trial demonstrated a benefit of consolidative allo-HSCT for high-risk patients.50 However, auto-HSCT for intermediate-risk patients with early relapse was not effective. Previous studies of the use of transplant in R/R ALCL had also suggested a benefit of allo-HSCT compared to auto-HSCT; however, treatment decisions around the perceived risk of relapse that influence the choice of type of transplant make direct comparison a challenge.47,71-74 Significantly, transplant-related mortality with allo-HSCT was high, with rates of ∼25% across studies. Of note, high-dose chemotherapy with auto-HSCT continues to be a standard approach for adults with R/R ALCL.75 Incorporating targeted therapy into reinduction for patients with R/R ALCL to achieve a CR while avoiding significant toxicity before transplant is now recommended over an intensive cytotoxic chemotherapy reinduction and may improve transplant-related toxicity. Reduced-toxicity conditioning regimens have also been shown to be safe and efficacious for R/R ALCL and should be considered as part of treatment planning.76,77
Recommendation
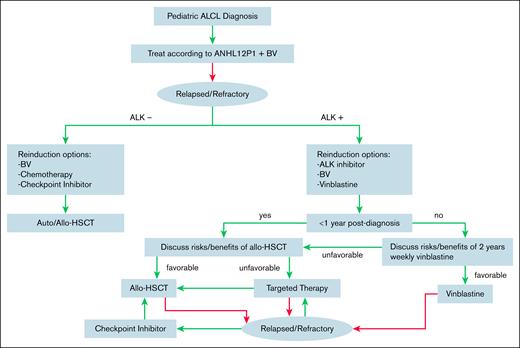
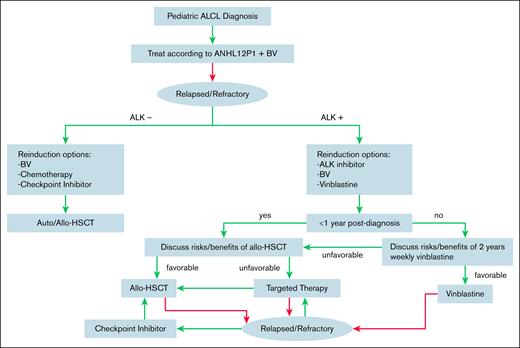
For R/R ALK+ ALCL, we recommend reinduction therapy with an ALK inhibitor, specifically a second- or third-generation ALK inhibitor if there is CNS involvement. BV can also be considered if not previously received as part of therapy. Discussions about the risks and benefits of HSCT are nuanced and should be individualized (Figure 1). Factors to consider include the timing of relapse, extent of disease involvement, donor availability, prior toxicities, response to and tolerability of targeted therapy, and willingness to continue on targeted therapy for a prolonged duration. For patients with late relapses, weekly vinblastine for 2 years should also be considered.
Treatment algorithm for pediatric patients with newly diagnosed or R/R ALCL.
For patients with early relapse who do not proceed with HSCT, there is little evidence to guide the duration of targeted therapy. We would recommend at least 2 years of targeted therapy and assessment of disease status with MRD measurement if available.
Special circumstances
CNS involvement
CNS involvement is rare in ALCL but can occur at initial diagnosis or at relapse and is associated with inferior survival. CNS involvement may manifest as neoplastic cells in cerebrospinal fluid, intracranial masses, or leptomeningeal disease. Additional CNS-directed chemotherapy is needed in these cases and may consist of systemic agents with CNS penetration (eg, high-dose methotrexate and cytarabine, as well as next-generation ALK inhibitors), intrathecal chemotherapy, and/or cranial radiation therapy (CRT).
In the ALCL99 study, 12 of 463 participants (2.6%) had CNS involvement at diagnosis, with the majority of these also having other systemic sites of disease.78 Chemotherapy regimens for these patients were variable but usually included high-dose methotrexate, high-dose cytarabine, and intrathecal chemotherapy, resulting in an initial CR in 75%. The 4 patients who received CRT, all of whom had an intracranial mass, became long-term survivors.
For frontline therapy of CNS+ ALCL, we recommend a chemotherapy regimen that includes intensive systemic and CNS-directed therapy, such as French-American-British/Lymphomes Malins B Group C therapy or ANHL12P1 with BV, with the addition of intrathecal chemotherapy during all cycles.
For relapse involving the CNS, treatment may include any of the modalities described above, followed by HSCT in most cases. Salvage therapy should be individualized based on prior and future treatment plans. Notably, vinblastine, BV, and crizotinib do not have adequate CNS penetration, so additional CNS-directed therapies are necessary.79 Second- and third-generation ALK inhibitors generally have excellent CNS penetration, tolerability, and efficacy in the treatment of R/R ALK-positive ALCL with CNS involvement.80 CRT should be considered once remission is obtained, before or in conjunction with an HSCT.
For systemic relapses of childhood ALCL not involving the CNS, CNS prophylaxis should be included in salvage regimens, either through repeated doses of intrathecal chemotherapy or by using a next-generation ALK inhibitor with CNS penetration.
cALCL
Cutaneous ALCL (cALCL) is very rare in children and differs significantly from systemic ALCL in clinical presentation, prognosis, and therapy. By definition, cALCL is confined to the skin, presenting as reddish skin nodules, ulcers, or plaques on the trunk and arms. It is localized in 80% of cases and behaves indolently. Although the neoplastic cells are morphologically similar to those of systemic ALCL and are usually CD30+, most cases are ALK−. Local therapies, such as surgical excision, low-dose radiation, or intralesional steroids, usually result in excellent outcomes.81-83 In adults with multifocal lesions, BV is the primary treatment.84
ALK− ALCL
The prognosis of ALK− ALCL in children remains unclear due to the small number of patients, but ALK negativity has been shown to be a negative prognostic factor in adults.33 Patients who were ALK− on the ALCL99 trial (16/420 [4%]) had similar outcomes to those who were ALK+.27 Studies in adult ALK− ALCL have demonstrated molecular heterogeneity, with mutations such as DUSP22 or TP63 associated with different outcomes.37 Typically, adults with ALK− ALCL receive multiagent anthracycline- and etoposide-containing regimens or BV with chemotherapy.26,85 Although ANHL12P1 excluded patients who were ALK− because 1 arm included an ALK inhibitor, ANHL12P1 with BV is our recommendation for frontline treatment of pediatric patients with ALK− ALCL. There are limited data on pediatric R/R ALK− ALCL, but treatments including BV, checkpoint inhibitors, and consolidation with auto-/allo-HSCT have been effective.30,64
Future directions
With the growing number of targeted therapies, there are numerous opportunities to improve the treatment of pediatric and adolescent patients with ALCL. The primary challenge lies in the design and execution of prospective clinical trials in a rare disease. International collaborations and trials open to patients of all ages may be helpful for patient accrual. Testing the safety and efficacy of the combination of BV and an ALK inhibitor in frontline treatment is an appealing approach that could improve efficacy while decreasing toxicity. Using a risk-adapted approach incorporating MDD/MRD into treatment allocation could also improve outcomes.
ALK inhibitors and BV have shown great responses in R/R ALK+ ALCL, but more needs to be learned about the durability of response. Combination therapy such as an ALK inhibitor and BV or ALK inhibitor and the phosphatidylinositol 3-kinase inhibitor duvelisib, which has been shown to reduce primary resistance and survival of persistent lymphoma cells in preclinical ALK+ ALCL models, may also provide more durable responses and is going to be studied in an upcoming trial (NCT07001384).86 Translating advances in the understanding of ALCL biology into targeted therapies and prospective trials will further improve outcomes for patients with ALCL.
Authorship
Contribution: All authors made substantial contributions to the conception and design of this work, assisted in drafting or substantive revision of the manuscript, and approved the submitted version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lianna J. Marks, Division of Pediatric Hematology, Oncology, Stem Cell Transplantation, and Regenerative Medicine, Department of Pediatrics, Stanford University School of Medicine, 750 Welch Rd, Suite 200, Stanford, CA 94304; email: marksl@stanford.edu.