Key Points
BRCA1/2 mutations play a significant role in the development of BIA-ALCL in women with BC reconstructed with textured implants.
The age-adjusted rate of developing BIA-ALCL for women with BRCA was 16 times the rate of BIA-ALCL without BRCA.
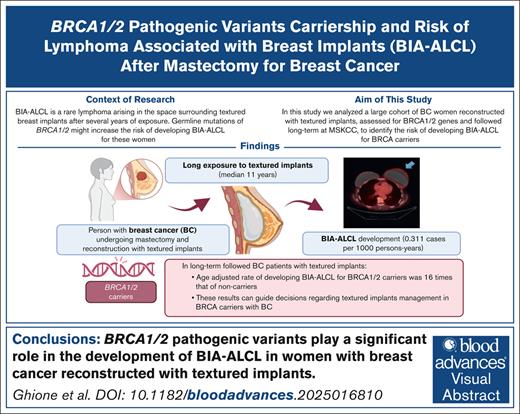
Visual Abstract
Breast implant–associated anaplastic large cell lymphoma (BIA-ALCL) is a type of T-cell lymphoma arising near textured breast implants. In a Dutch population, a higher prevalence of BRCA1/2 was found in BIA-ALCL. We analyzed the risk of BIA-ALCL occurrence related to BRCA in a large population of women with implants followed after breast cancer (BC) mastectomy. We compared the prevalence of BRCA1/2 between women from a large cohort of patients with BC who did and did not develop BIA-ALCL after reconstruction with textured implants. Hazard ratios (HRs) of developing BIA-ALCL were estimated using Cox regression. We also conducted a case-control study. Of 520 patients with BC tested for BRCA, the age-adjusted rate of developing BIA-ALCL for women with BRCA was 16 times the rate of BIA-ALCL among women without BRCA (95% confidence interval [CI], 3.6-76.1; P < .0003). Carrying bilateral implants (HR, 3.9; 95% CI, 0.4-32.7), chemotherapy (HR, 0.95; 95% CI, 0.2-4.2), and radiotherapy (HR, 0.37; 95% CI, 0.04-3.1) were not associated with BIA-ALCL. We also conducted a case-control study with 13 BIA-ALCL patients matched 1:3 with 39 controls. We used a complete enumeration of Bernoulli probability to rule out a nonassociation of BRCA with BIA-ALCL (P = .0002). In this study, we defined the role of BRCA1/2 mutations as a risk factor in developing BIA-ALCL in patients with BC. These results will help women undergoing breast reconstruction or with textured implants in place.
Introduction
Breast implant–associated anaplastic large cell lymphoma (BIA-ALCL) is a rare subtype of T-cell lymphoma, recently recognized within the World Health Organization classification of lymphoid malignancies1 and arising near breast implants, either as a fluid collection or as a mass. The latest update on BIA-ALCL patients by the US Food and Drug Administration (FDA) in June 2023 included a total of 1264 patients and 63 deaths related to this lymphoma. Based on a previous version of this report in 2019, several national agencies worldwide, including the FDA itself, had recalled from the market one of the most commonly implanted textured surface devices whose exposure has been related to BIA-ALCL.2,3 This recall increased public awareness of this disease in the United States, significantly raising concerns for women with textured implants in place and their treating physicians. It also identified an unmet need to more precisely assess risk estimates to drive clinical decisions by physicians and patients on implant removal or the type of reconstruction after mastectomy.
This study sought to determine whether germ line mutations of the hereditary cancer susceptibility genes play a role in the lymphomagenesis of BIA-ALCL and specifically whether BRCA germ line mutation carriership represents a risk factor for developing BIA-ALCL in women with textured surface breast implants.
This study was developed based on 3 premises. First, our collaborators from the Netherlands Cancer Institute have reported a higher prevalence of BRCA1/2 germ line pathogenic variants (pv), traditionally associated with breast and ovarian cancer, in patients with BIA-ALCL (26.7%) than the general Dutch population, estimated at 0.5%, suggestive of BRCA1/2 as a risk factor.4 Second, we have published in the Journal of Plastic and Reconstructive Surgery (JPRAS; Cordeiro et al)5,6 an estimate of the risk of BIA-ALCL based on a prospective cohort of 3546 women with textured breast implants used in reconstruction after mastectomy for breast cancer (BC), in which the incidence of BIA-ALCL was 1 patient per 322 women exposed (“JPRAS cohort”), with an incidence rate of 0.311 patients per 1000 person-years (95% confidence interval [CI], 0.12-0.49). This risk was much higher than previously reported, with all other published studies estimating the incidence of BIA-ALCL using an approximated denominator of women exposed from population estimates and manufacturers’ sales records and not following women long term. However, our high-risk estimate for BIA-ALCL might be related to characteristics of our population that have not yet been identified, including a relatively high prevalence of germ line mutations. For example, BRCA1/2 pv are more prevalent in some ethnic groups, such as Ashkenazi Jews (AJ), than in the general population,7,8 with a BRCA1-specific mutation present in ∼1% of the AJ.9 In New York City, there are large communities of AJ, especially in Brooklyn and Long Island. Third, at Memorial Sloan Kettering Cancer Center (MSKCC), most women with BC receive an assessment of the BRCA mutations either directly through traditional clinical assay in patients at a higher genetic risk or indirectly through our sequencing platform MSK-IMPACT (integrated mutation profiling of actionable cancer targets). This tumor sequencing method includes the analysis of normal DNA to exclude germ line mutations from the tumor results. Approximately 4000 women with BC underwent MSK-IMPACT testing.
In this study, we determined the risk associated with BRCA1/2 pv carriership in a large cohort of patients with breast implants after mastectomy for BC. This information will be essential for women carrying a BRCA1/2 germ line mutation and their treating physicians when deciding on breast reconstruction after BC surgery and for those who have breast implants in place.
Methods
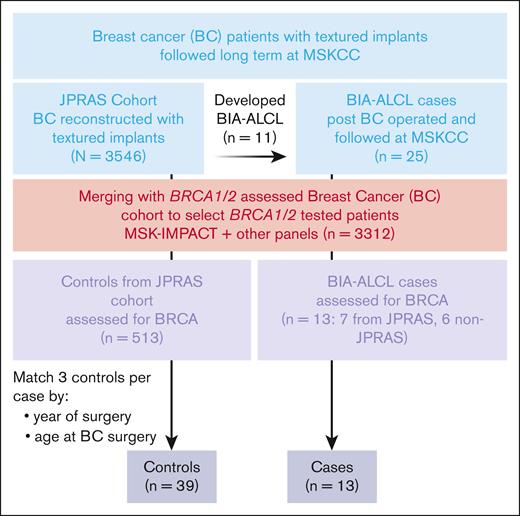
After obtaining institutional review board approval from the MSKCC institutional review board, we referenced our institutional databases to identify the women from the JPRAS cohort who had been tested for BRCA1/2 pv. We performed Cox regression analysis on this population to evaluate the hazard ratio (HR) of developing BIA-ALCL related to the carriership of BRCA1/2 pv (Figure 1).
We then conducted a case-control study using as cases all the BIA-ALCL seen at MSKCC, including those who developed the disease within the JPRAS cohort and the non-JPRAS patients meeting similar criteria of the JPRAS cohort, but being operated on by other surgeons in the division. We used the rest of the JPRAS cohort, which did not develop BIA-ALCL, as control. The women in the JPRAS cohort were enrolled in a prospective observational study to evaluate long-term complications of breast implants and informed consent had been waived for that study and the present analysis as well. Eligibility criteria for the patients in the case-control study were (1) undergoing mastectomy and reconstruction with textured breast implants after diagnosis of BC, (2) being followed long term at our institution, and (3) having a confirmed diagnosis of BIA-ALCL by our pathology department.
Patient populations
Data and DNA samples for this study were drawn from databases of overlapping cohorts of patients from MSKCC. The first database (n = 3312) included people with BC treated and followed at MSKCC and analyzed for germ line mutations including BRCA1/2 pv. This database included both women assessed for germ line BRCA1/2 pv using clinical panels, who were diagnosed and assessed for BC before 2012, and women getting a germ line mutations assessment in the context of our tumor plus normal paired targeted sequencing platform, MSK-IMPACT.
The second cohort is the “JPRAS cohort,”5 from our previous study on the incidence of BIA-ALCL in women with breast implants who were followed long term after surgery for BC. This group of 3546 women with BC underwent reconstruction with textured breast implants after mastectomy for BC from 1992 to 2017 at MSKCC by the plastic surgeon P.C. Patients were followed at MSKCC for a median of 8.1 years, and the risk of BIA-ALCL was estimated to be 1 of 322 women exposed with the latest update. Women in that cohort had a median age of 48 years at the time of surgery. The baseline BC histology was lobular carcinoma in 685 (19.3%), ductal carcinoma in 2543 (71.7%), other types of BC in 60 (1.7%), and unknown in 258 (7.2%). BC management, other than surgery, had been chemotherapy only in 1025 (28.9%), radiotherapy only in 287 (8.1%), chemotherapy plus radiotherapy in 714 (20.1%), and none in 1520 (42.9%).
Evaluation of the AJ-related BRCA germ line mutations
AJ founder mutations (BRCA1, c.68_69delAG and c.5266dupC; BRCA2, c.5946delT)10 have been selected in our database. The proportion of BRCA1/2 mutations attributable to this population, which might constitute a bias for the generalization of the BRCA1/2 data to the general BC population with textured implants, could be unequivocally identified, given that these 3 founder mutations are thought to account for the vast majority of the BRCA1/2 mutations (∼96%) in the AJ population.
Statistical methods
Descriptive statistics, including means and standard deviations of quantitative variables and frequencies of qualitative variables, are reported. Within the JPRAS cohort, we compared the prevalence of BRCA1/2 pv between patients with BC with textured implants followed at MSKCC who did and did not develop BIA-ALCL. HRs of developing BIA-ALCL were estimated using Cox regression. Because death was hypothesized to be a competing risk of developing BIA-ALCL, we calculated the subdistribution hazard of developing BIA-ALCL accounting for death using the Fine-Gray model (proportional subdistribution hazards model). Median follow-up time and the interquartile range were calculated using the reverse Kaplan-Meier method.
We also conducted a matched case-control study using as cases all the women diagnosed as having BIA-ALCL operated on and followed long term at MSKCC, including those not originally in the JPRAS cohort, and compared them with controls from the JPRAS cohort. Each patient was matched with 3 controls individually based on the year of surgery (±3 years) and age at BC surgery (±3 years). We calculated the presence of an association between BRCA mutations and BIA-ALCL with the likelihood ratio test and the probability of observing ≥5 BRCA+ patients with a complete enumeration of Bernoulli probability. The analysis was performed with the statistical programs R and SAS.
Analysis of other germ line mutations in the patients with BIA-ALCL assessed by MSK-IMPACT
To explore the presence of additional germ line mutations in patients developing BIA-ALCL, we extracted germ line mutational data from the MSK-IMPACT analysis of patients with BIA-ALCL.
The MSK-IMPACT is a hybridization capture-based next-generation sequencing panel that can detect all protein-coding mutations, copy number alterations, and selected promoter mutations and structural rearrangements in several cancer-associated genes. In this context, a key feature of the process is using normal controls matched to the patient’s tumor to enable us to distinguish between germ line and tumor mutations. The blood component of the MSK-IMPACT assay is analyzed for germ line variants in 90 cancer susceptibility genes.11-14
We reanalyzed the binary alignment map files from 8 patients with BIA-ALCL analyzed using IMPACT-Heme at the time of BIA-ALCL occurrence to extract the germ line genetic information with anonymized identifications (IDs). These were created with a deterministic hash function that cannot convert them back to the recognizable IDs and re-ID.
Results
Population
We enrolled 520 women from the JPRAS cohort who were tested for BRCA1/2 pv (7 patients with BIA-ALCL). For the case-control part of the study, we also included additional 6 patients with BIA-ALCL tested for BRCA, operated on, and followed at MSKCC, but outside of the JPRAS cohort. Characteristics of this population are presented in Table 1.
We first analyzed the entire JPRAS cohort, including in the denominator all women with BC and macrotextured implants regardless of whether they had previous BRCA testing or not.
We initially found that BRCA1/2 testing was more frequent in patients than healthy controls. Hence, we ran into singularity problems when restricting to the BRCA1/2 tested-only population. This increased risk of BIA-ALCL in the BRCA1/2 tested population, which we assume is the same in patients and healthy controls, is explainable by the nature of clinical testing for BRCA, which is offered in patients who have familiarity with BC or are at a higher risk of secondary malignancies. Nonetheless, we were able to test for independence of the BRCA1/2 variable using the likelihood ratio test in the conditional logistic approach (parameter estimate, 11.09; P = .0009).
JPRAS cohort Cox regression analysis and AJ mutations evaluation
A total of 520 patients in the JPRAS cohort were tested for BRCA1/2. All the patients diagnosed as having BC before 2012 were tested with clinical panels focused on the BRCA genes; after that date, some were also tested for BRCA in the context of the MSK-IMPACT targeted sequencing analysis. In this group, with a median follow-up of 138 months (standard error, 1.24; interquartile range, 98-206), 7 patients developed BIA-ALCL. The prevalence of BRCA1/2 pv in the JPRAS cohort was 8.3% (n = 43 patients).
In the JPRAS cohort subgroup tested for BRCA1/2 pv, 5 of 43 BRCA+ patients carried an Ashkenazi founder mutation of BRCA: 1 BRCA1 c.68_69delAG, 1 BRCA1 c.5266dupC, and 3 BRCA2 c.5946delIT; none of these 5 patients developed BIA-ALCL.
Within the cohort of 520 patients, the age-adjusted rate of developing BIA-ALCL for women with BRCA1/2 mutations was 16 times the rate of BIA-ALCL among women without BRCA1/2 (95% CI, 3.6-76.1; P < .0003).
Single-variable Cox regression analyses were performed for BRCA1/2 and other clinically relevant variables including carrying bilateral vs unilateral implants, exposure to previous chemotherapy or radiotherapy, age at surgery for BC, year of surgery, BC histology, and staging characteristics. BRCA1/2 carriership, age at BC surgery, and infiltrating carcinoma (compared with in situ neoplasia) were possibly associated with the development of BIA-ALCL and were then included in a multivariable Cox regression analysis. Results are presented in Table 2. In the regression analysis, having a history of infiltrating carcinoma resulted as a possible protective trait to the development of BIA-ALCL; hence, to control for death as a competing risk, we additionally calculated cause-specific HR (calculated censoring death) and conducted Fine-Gray analysis for BIA-ALCL occurrence (Table 3). In this analysis, the HR of having an infiltrating carcinoma was 0.21 (95% CI, 0.05-1; P = .048).
Case-control study
Of 31 patients with BIA-ALCL after BC seen at MSKCC so far, 25 patients had similar characteristics to the JPRAS group.15 Six patients were excluded from this analysis: 4 had implants for cosmetic reasons not after BC, 2 were seen in second opinion only, and we were not able to review their pathology to confirm the case. Eleven patients were part of the JPRAS cohort and 14 were not in the cohort (ie, were seen and operated on by other surgeons than P.C.). Their characteristics were as follows: (1) underwent mastectomy after BC and reconstruction with textured breast implants; (2) were followed long term at MSKCC, with a median follow-up of 190 months (interquartile range, 158-224); (3) were operated on for implant removal and capsulectomy at MSKCC at BIA-ALCL occurrence; and (4) were assessed periodically for the development of breast implant complications (including BIA-ALCL). Overall, 13 of 25 of these patients (7 from JPRAS and 6 non-JPRAS) were assessed for BRCA1/2 mutations.
We considered these 13 BRCA-tested patients with BIA-ALCL and compared them with the 513 patients at risk of developing BIA-ALCL and BRCA tested in the JPRAS cohort. The 13 patients with BIA-ALCL matched 1:3 with 39 controls as described in the “Methods,” controlling for age at BC diagnosis (±3 years) and year of surgery (±3 years). One patient did not have 3 matches so for that patient the selection was expanded to ±5 years. Baseline characteristics of the 2 groups compared are presented in Table 4.
We calculated the probability of observing ≥5 BRCA1/2+ patients in the BIA-ALCL cases with an at-risk table using a complete enumeration of Bernoulli probabilities (P = .0002; Table 5). The clinical characteristics of the patients with BIA-ALCL and BRCA1/2 mutation subtypes are presented in Figure 2.
Patients with BIA-ALCL who were tested for BRCA and their lag time (years of exposure) between BC mastectomy and reconstruction with textured implants and BIA-ALCL development. The table on the right shows the MD Anderson TNM staging at BIA-ALCL diagnosis for each patient. TNM, tumor, node, metastasis.
Patients with BIA-ALCL who were tested for BRCA and their lag time (years of exposure) between BC mastectomy and reconstruction with textured implants and BIA-ALCL development. The table on the right shows the MD Anderson TNM staging at BIA-ALCL diagnosis for each patient. TNM, tumor, node, metastasis.
Analysis of other germ line mutations in the patients with BIA-ALCL assessed by MSK-IMPACT
Patients with BC getting tested for BRCA in the context of the MSK-IMPACT analysis demonstrated a BRCA1/2 pv prevalence of 5.3%, whereas patients clinically assessed for BRCA on a restricted panel had a higher prevalence of BRCA mutations (7.8%). Testing for BRCA in the JPRAS cohort before 2012 was performed mostly at diagnosis of BC using the clinical genetic platforms in patients who were deemed at a higher risk of carrying those mutations because of a family history of BC or other cancers. Hence, the population tested for BRCA in the JPRAS cohort might be at a higher risk of secondary cancers or harbor other germ line mutations. We then reanalyzed 8 patients with BIA-ALCL who were tested for tumor mutations using IMPACT-Heme at the time of BIA-ALCL occurrence to look for germ line mutations other than BRCA. As a group, we could identify the presence of 1 additional ATM germ line mutation in these patients with BIA-ALCL, in addition to the previously known BRCA1/2 mutations.
Discussion
Our results indicate that germ line BRCA1/2 pv might play a role in the development of BIA-ALCL in women with previous BC exposed to implants. Owing to the unique characteristics of our cohort, we were able to estimate the HR of developing BIA-ALCL related to BRCA1/2 pv for patients after mastectomy for BC carrying textured breast implants.
To our knowledge, this is the first prospectively monitored cohort in which women with implants followed long term were assessed for germ line mutations in relation to the risk of developing BIA-ALCL. This is also the first study to have assessed germ line DNA alterations of patients with another cancer who are at risk of developing lymphoma. For the first time, we were also able to evaluate multiple possible confounders of the relationship between BRCA1/2 pv and development of lymphoma, for example, previous exposure to chemotherapy or radiotherapy, histology of BC at diagnosis, death as a competing risk, and the extent of surface exposure (unilateral vs bilateral implants). None of the other interrogated variables demonstrated a meaningful relationship with the development of BIA-ALCL. However, it is important to acknowledge that, because of the very small number of patients with BIA-ALCL, the subgroup analyses for additional risk factors, as well as the detection of additional germ line mutations of relevance, are underpowered to draw definite conclusions.
The American Society of Plastic Surgeons estimated that 1 862 506 breast augmentations were performed worldwide in 2018.16 In the United States, 304 181 breast implant augmentation procedures and 157 740 breast reconstructive procedures (122 527 involving breast implants) were performed in 2023, in a rising trend.17
The highly textured surface implants implicated in the development of BIA-ALCL were withdrawn from the US, European Union, and Brazilian markets in 2019. The percentage of implantation of highly textured surface devices in the United States was 12.7% in 2017 and may have recently decreased below 5% after the recall in March 2019.17 However, a study of implant sales suggests that the ratio of macrotextured to smooth implantations before the recall was inverted in Europe compared with the United States.16 Based on these ratios, we estimate that the prevalence of people living with textured surface devices in place may range between 2 and 5 million.
Data from large databases put together by the FDA (1030 participants)3 and the French Lymphoma Study Association (88 participants)18 seem to show that 50% to 52% of the BIA-ALCL cases occurred in women who underwent reconstruction after mastectomy for BC and 48% to 50% in women with cosmetic augmentation. However, national database studies in Europe and North America demonstrate that approximately 62% to 75% of breast implants are inserted for cosmetic reasons and 25% to 38% for reconstruction after mastectomy.19-22 Although these data are extremely approximate, they seem to indicate a higher incidence of BIA-ALCL in women with reconstruction after mastectomy for BC. The implications of the BC (histology and stage) and its management (radiotherapy/chemotherapy) in the lymphomagenesis may be marginal given that BIA-ALCL occurs after a median of 11 years after mastectomy, and most patients are BC free after that initial surgery.23 The finding in multivariate analysis that patients with an infiltrative carcinoma might be potentially “protected” from developing BIA-ALCL was mitigated when controlling for death as a competing risk of the development of the lymphoma (the CI was nonsignificant). This is also underlined by the survival curve of this patient subgroup (Figure 2).
The risk of BIA-ALCL for women with breast implants has been difficult to determine because most studies estimating the numbers of exposed and at risk have approximated the denominator of women exposed from the manufacturers’ sales records or population estimates.19,24-29 In the JPRAS cohort,5 the occurrence of BIA-ALCL did not seem to be related to the type of BC or previous treatment with radiotherapy or chemotherapy. Hence, it seems clear that other factors, such as genetic predisposition, might affect the susceptibility of some people to develop BIA-ALCL. In this cohort, we detected the highest incidence of BIA-ALCL described so far: of 3546 people with implants, 11 women had developed BIA-ALCL (incidence rate of 0.311 patients per 1000 person-years; 95% CI, 0.12-0.49). We believe our much higher risk than previously described has been identified because of a long and carefully conducted follow-up of these women.24,28,30 However, our population might also be enriched with people carrying germ line mutations, such as BRCA1/2, leading to increased genome instability and possibly lymphomagenesis. As mentioned earlier, in New York city, there are large communities of AJ families. In a large population study, 10% of Jewish females who had been given a diagnosis of BC before the age of 40 years carried one of the BRCA mutations.31 In a large study conducted at MSKCC10 in AJ families, patients diagnosed as having BC at the age of <50 years were found to have a higher prevalence of BRCA1/2 mutations than patients with BC who were diagnosed at the age of >50 years (21.1% vs 6.9%; P = .003). AJ founder mutations, which could have been higher in our population than others, were detected in a minority of the patients, but not in those patients who developed BIA-ALCL.
The proteins encoded by the BRCA1/2 genes are involved in DNA repair mechanisms: their mutations lead to chromosome instability and accumulation of more mutations, raising the hypothesis that BRCA1/2 could also be involved in this type of lymphomagenesis. A large cohort study performed at MD Anderson Cancer Center32 involving 1072 subjects with BRCA pv identified a total of 1177 patients with cancer, classifiable in 30 different cancer subtypes; however, no BIA-ALCL was included in these patients. Our collaborators from the Netherlands Cancer Institute analyzed 49 patients with BIA-ALCL included in the Dutch national pathology database.4 In this cohort, 6 patients had germ line BRCA1/2 pv (12.2%). Of the 15 patients with BIA-ALCL after BC reconstruction, 4 carried BRCA1/2 pv (26.7%). The authors have estimated, from the Dutch Breast Cancer cohort, an expected prevalence of BRCA1/2 mutations in this group of 5.1%, indicating a higher incidence of these mutations in the BIA-ALCL cohort. Separate references were obtained after BC and cosmetic cases.
The number of patients in our cohort is very small, which is certainly related to the rarity of the disease, and this might affect the reliability of our estimate. The 95% CI for the HR calculated in our analysis is statistically significant but quite wide, indicating that there is an association of BRCA1/2 pv with BIA-ALCL development, but the real weight of this association is still to be defined.
The results generated by this analysis will be useful in guiding decisions regarding textured implant placement in women with BC and BRCA1/2 mutations after mastectomy, but also potentially for women with these mutations with implants in place or with those considering prophylactic mastectomy because of being carriers of BRCA1/2 pv.
Acknowledgments
The authors acknowledge the members of the Molecular Diagnostics Service in the Department of Pathology at Memorial Sloan Kettering Cancer Center.
This research was funded by National Institutes of Health/National Cancer Institute grant R03CA26743 (principal investigator, P.G.). It was also partially funded by National Institutes of Health/National Cancer Institute Cancer Center support grant P30CD008748.
Authorship
Contribution: P.G., M.A., S.H., D.M., M.B.T., D.d.J., and F.v.L. designed the study; P.G., J.V., D.M., and V.S. performed the analysis; P.C. kept the records of the Journal of Plastic and Reconstructive Surgery database and performed the surgeries; S.H., P.G., and P.C. followed the patients; D.M. and J.V. performed the germ line testing; C.V., J.P., A.C., and N.G. helped with data processing; M.A., A.C., and A.D. performed the pathological analysis; P.G. wrote the manuscript; and all authors reviewed and approved the final draft of this manuscript.
Conflict-of-interest disclosure: P.G. reports research support from AbbVie/Genmab and consulting roles with Regeneron, Ipsen, AbbVie/Genmab, and ADC Therapeutics. P.C. reports research support from Allergan, Inamed, and Acelity, outside of the submitted work. A.D. reports research funding from Roche/Genentech and consulting roles for AstraZeneca. G.S. reports research support from Janssen, Ipsen, AbbVie, Genmab, Genentech, and Nurix, and consulting roles with Roche/Genentech, Janssen, Novartis, Epizyme, Genmab, Bristol Myers Squibb, BeiGene, Incyte, Ipsen, AbbVie, Kite/Gilead, Loxo/Lilly, Merck, Orna Therapeutics, Nurix, Pfizer, Modex, and Treeline Biosciences. S.H. reports research support from ADC Therapeutics, Affimed, Celgene, Corvus, CRISPR Therapeutics, Daiichi Sankyo, Kyowa Hakko Kirin, Takeda, Seattle Genetics, Treeline Biosciences, Trillium Therapeutics, and Secura Bio, and consulting roles with Arvinas, Corvus, Daiichi Sankyo, Dren Bio, Johnson & Johnson Medicine/Janssen Research & Development, Kyowa Hakko Kirin, March Biosciences, Ono Pharmaceutical, Pfizer, Secura Bio, Shoreline Biosciences, Inc, Symbio, and Takeda. The remaining authors declare no competing financial interests.
Correspondence: Paola Ghione, Memorial Sloan Kettering Cancer Center, 530 East 74th St, New York, NY 10021; email: ghionep@mskcc.org.
References
Author notes
Deidentified are available on request after case-by-case consideration by the corresponding author, Paola Ghione (ghionep@mskcc.org).