With extended treatment, responses with rilzabrutinib were durable as a monotherapy and with the use of concomitant ITP medication.
Oral rilzabrutinib was well tolerated, with no treatment-related serious events or deaths.
Visual Abstract
Immune thrombocytopenia (ITP) is an autoimmune disease associated with autoantibody-mediated platelet destruction and impaired platelet production, resulting in thrombocytopenia and a predisposition to bleeding. The ongoing, global phase 1/2 study showed that rilzabrutinib, a Bruton tyrosine kinase inhibitor specifically developed to treat autoimmune disorders, could be an efficacious and well-tolerated treatment for ITP. Clinical activity, durability of response, and safety were evaluated in 16 responding patients who continued rilzabrutinib 400 mg twice daily in the long-term extension (LTE) study. At LTE entry, the median platelet count was 87 × 109/L in all patients, 68 × 109/L in those who had rilzabrutinib monotherapy (n = 5), and 156 × 109/L in patients who received concomitant ITP medication (thrombopoietin-receptor agonists and/or corticosteroids, n = 11). At a median duration of treatment of 478 days (range, 303-764), 11 of 16 patients (69%) continued to receive rilzabrutinib. A platelet count of ≥50 × 109/L was reported in 93% of patients for more than half of their monthly visits. The median percentage of LTE weeks with platelet counts ≥30 × 109/L and ≥50 × 109/L was 100% and 88%, respectively. Five patients discontinued concomitant ITP therapy and maintained median platelet counts of 106 × 109/L at 3 to 6 months after stopping concomitant ITP therapy. Adverse events related to treatment were grade 1 or 2 and transient, with no bleeding, thrombotic, or serious adverse events. With continued rilzabrutinib treatment in the LTE, platelet responses were durable and stable over time with no new safety signals. This trial is registered at www.clinicaltrials.gov as #NCT03395210 and www.clinicaltrialsregister.eu as EudraCT 2017-004012-19.
Introduction
Immune thrombocytopenia (ITP) is an acquired autoimmune blood disease with an estimated global prevalence of ∼10 to 23 in 100 000 people.1-7 ITP is diagnosed when there is an isolated platelet count <100 × 109/L and is caused by a combination of immune-mediated platelet destruction and impaired platelet production, leading to thrombocytopenia that increases the risk of bleeding along with a predisposition to reduced quality of life (QOL).8-10 Mechanisms associated with ITP include pathogenic antiplatelet autoantibodies that target surface glycoproteins αIIbβ3 [GPIIbIIIa], GPIa/IIa, and GPIb-IX-V.11,12 This may result in downstream events including phagocytosis of opsonized platelets upon binding to macrophage Fcγ receptors (FcγRs), platelet lysis through the classical complement pathway, and/or impaired megakaryocyte platelet production.11-14 Some patients may also have autoreactive T cells resulting in toxicity to platelets and/or megakaryocytes.12,15
Bruton tyrosine kinase (BTK) is a potentially important target in ITP due to its wide expression in B cells and innate immune cells and its role in B-cell maturation, antibody production, regulation of innate inflammatory machinery NLRP3 inflammasome, and FcγR-mediated signaling pathways in immune-mediated diseases.16-19 Inhibition of BTK may affect inflammatory and autoimmunity pathways leading to decreased autoantibody production and macrophage function (via FcγR), as well as reduction in FcεR-induced mast cell degranulation, granulocyte migration, and mediator release.16,17 Rilzabrutinib is a potent oral, reversible BTK inhibitor that has the potential to act on multiple immunological mechanisms (eg, inhibition of B-cell activation and prevention of FcγR-mediated phagocytosis) and induces rapid and sustained anti-inflammatory effects.20-22
In an international, adaptive, open-label, dose-finding phase 1/2 clinical trial to evaluate a safe and effective rilzabrutinib dose in ITP, patients were treated with different doses (200 mg once daily, 400 mg once daily, 300 mg twice daily, and 400 mg twice daily) of oral rilzabrutinib for 24 weeks.23 In patients with ITP with a median duration of disease of 6 years and a median of 4 prior ITP therapies, rilzabrutinib was active and associated with only grade 1 or 2 transient adverse events (AEs) at all dose levels. After a median of 167.5 days of treatment, 24 of 60 patients (40%) in the intent-to-treat population and 18 of the 45 patients (40%) who initiated rilzabrutinib 400 mg twice daily, which was identified as the dose for further testing, met the primary platelet response end point (defined as ≥2 consecutive platelet counts [separated by ≥5 days] of ≥50 × 109/L and an increase from baseline of ≥20 × 109/L without the use of ITP rescue medication in the 4 weeks before the latest elevated platelet count). During the main 24-week treatment period, rilzabrutinib showed rapid and durable clinical activity that improved with longer treatment.
We report results from a long-term extension (LTE) study conducted to investigate whether patients who responded during the main treatment period maintained platelet responses throughout the extension period, assess whether long-term exposure to rilzabrutinib was associated with new safety signals, address the stability of response between patients who received rilzabrutinib with and without concomitant ITP medication, and explore the potential for discontinuation of concomitant ITP medication.
Methods
Study design
Results from the initial dose-finding stage of this phase 1/2 clinical trial have been published previously, in which the study design was described in detail (clinicaltrials.gov identifier: NCT0339521024; EudraCT 2017-004012-1925).23 The trial was conducted in accordance with the principles of the Declaration of Helsinki and the International Council for Harmonisation E6 requirements. The protocol and informed consent documents were approved by the ethics committee at each participating institution. All patients provided written informed consent.
Study population
Eligible patients with ITP for the initial study were aged 18 to 80 years with platelet counts of <30 × 109/L on 2 occasions ≥7 days apart within 15 days before trial entry. Patients had received ≥1 previous ITP therapy (which might include splenectomy) but had inadequate response to their most recent therapy at study entry. Stable concomitant ITP medication with a corticosteroid (CS) or thrombopoietin-receptor agonist (TPO-RA) with ≤10% dose change within 2 weeks before rilzabrutinib initiation was allowed unless there were safety concerns related to their use. If any rescue medication was used to treat platelet count deterioration that the investigator felt put the patient at risk of a serious AE, rilzabrutinib was discontinued, and the patient was removed from the study. To be eligible to participate in the LTE portion of the study, patients were required to demonstrate platelet counts ≥50 × 109/L or 30 × 109/L plus a doubling of the baseline count for ≥50% of the patient’s final 8 weeks of rilzabrutinib’s main 24-week treatment period.
End points and assessments
The primary efficacy end point for the main study period was defined as ≥2 consecutive platelet counts (separated by ≥5 days) of ≥50 × 109/L and an increase from baseline of ≥20 × 109/L without the use of rescue medication for ITP in the previous 4 weeks before the latest elevated platelet count (ie, latest platelet count that fulfilled response criteria). Post hoc analyses using descriptive statistics during the LTE included safety along with efficacy end points that included the percentage of weeks with platelet counts that increased ≥20 × 109/L above baseline, were ≥30 × 109/L , or ≥50 × 109/L; the proportion of patients with a platelet count of ≥100 × 109/L at any time; and ≥50 × 109/L for ≥50% of monthly/quarterly visits in the last 12 months of treatment. QOL was measured as an exploratory end point through the LTE period. The effects on QOL were measured using the Euro-QoL 5-Dimension Visual Analog Scale (EQ-5D VAS) evaluating patient-reported outcomes on a scale of 0 (worst) to 100 (best).26,27 Test results from the general population have shown a pooled mean of 91 (95% confidence interval, 89-92).27,28
After the initial 6 months of weekly LTE visits, patients had monthly assessments for an additional 6 months (total 12 months of LTE) and then every 3 months until they were no longer on study. AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0); the relationship to the study drug was assessed per the investigator’s judgment. The proportion of patients with grade ≥2 bleeding events and bleeding symptoms based on the ITP Bleeding Scale29 were also examined. Descriptive summaries were used for the efficacy assessments.
Results
Patients and treatment
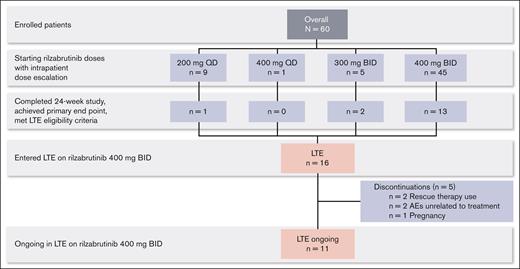
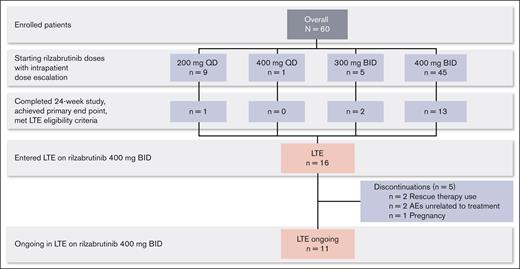
Sixteen patients who completed the 24-week main treatment period and met LTE eligibility criteria continued in the LTE on the 400 mg twice daily rilzabrutinib oral dose (Figure 1; database lock 9 August 2021). For the original main treatment period (before LTE dosing at 400 mg twice daily), of those 16 patients, 1 had started on rilzabrutinib at 200 mg daily, 2 at 300 mg twice daily, and the majority (13 patients) had initiated and continued 400 mg twice daily. At a median duration of treatment for the main study period plus the LTE of 478 days (range, 303-764), 11 patients were still ongoing treatment in the LTE, and 5 discontinued. Discontinuations were due to rescue therapy use (as required per the study protocol) in 2 patients, AEs unrelated to treatment per the investigator’s judgment in 2 patients (grade 4 thrombocytopenia and grade 3 worsening migraine), and pregnancy in 1 patient. For the 2 patients who discontinued rilzabrutinib due to rescue medication, both had been receiving rilzabrutinib monotherapy for ≥6 months during the LTE before rescue medication use that was given at platelet counts of 8 × 109/L and 73 × 109/L. After the initial 24-week main treatment period, the median treatment duration on the LTE was 309 days (range, 115-595). Rilzabrutinib treatment compliance, assessed by the actual total cumulative dose (mg) taken divided by the expected cumulative dose (mg), was a mean of 98% (standard deviation [SD], 2%) during the LTE period.
At baseline, the median age of the 16 patients in LTE was 49 years (range, 22-65), and 56% were female (Table 1). The median platelet count at LTE entry was 87 × 109/L (range, 16 × 109/L to 321 × 109/L). These patients had ITP for a median duration of 4.3 years (range, 0.5-18.4) and had received a median of 3 (range, 1-9) unique prior ITP therapies, including 3 patients (19%) with prior splenectomy. During the LTE, 5 patients (31%) received rilzabrutinib monotherapy, and 11 (69%) received concomitant ITP medication (7 patients had CS, 2 TPO-RA, and 2 both concomitant CS and TPO-RA).
Efficacy
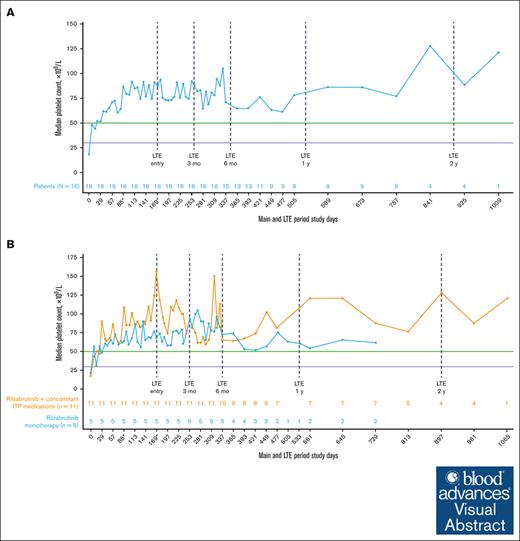
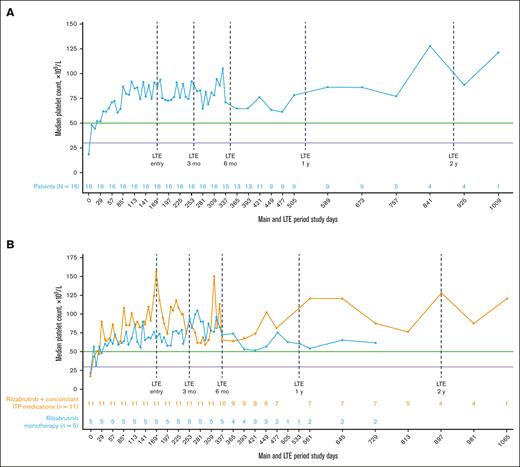
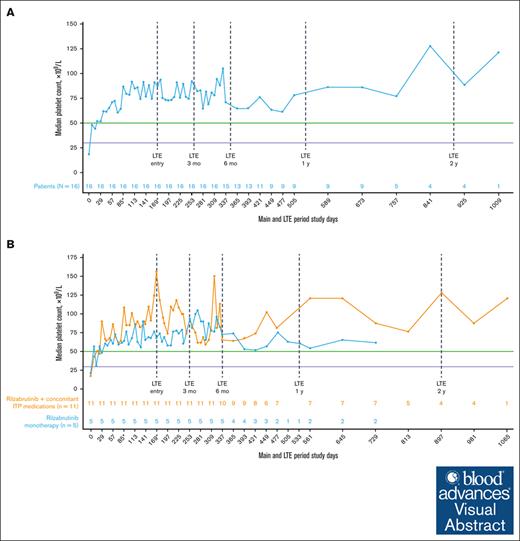
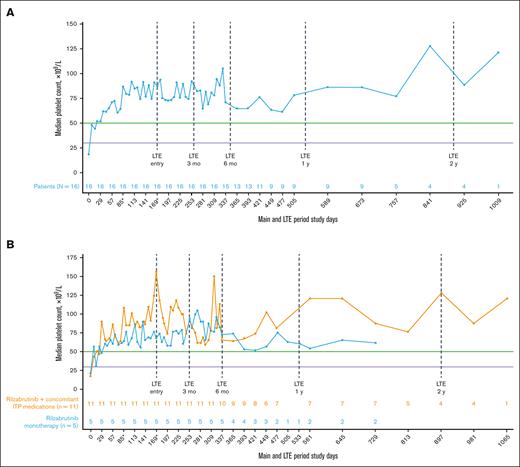
All 16 patients who met eligibility for and entered the LTE had achieved the primary efficacy end point during the main treatment period. Throughout the main study and LTE periods, the median platelet response was maintained above the 50 × 109/L threshold level (Figure 2A), regardless of the use of concomitant ITP medication (Figure 2B; Table 2). In the LTE, the median percentages of weeks with a platelet count of ≥30 × 109/L, ≥30 × 109/L along with ≥20 × 109/L over baseline, and ≥50 × 109/L were 100%, 97%, and 88%, respectively. At 3 and 6 months in the LTE, patients maintained platelet count thresholds that had increased ≥20 × 109/L above baseline or were ≥30 × 109/L or ≥50 × 109/L for at least 85% of weeks, irrespective of receiving rilzabrutinib with or without concomitant ITP medication (Table 3). The median change in platelet counts from baseline were higher at LTE entry in patients who received concomitant ITP medication (129 × 109/L) relative to rilzabrutinib monotherapy (47 × 109/L), but the median changes from baseline at LTE 3 months (66 × 109/L to 69 × 109/L) and LTE 6 months (46 × 109/L to 53 × 109/L) were similar between patient groups (Figure 2B).
Median platelet counts in patients who continued rilzabrutinib 400 mg BID in the LTE. (A) All 16 patients in LTE and (B) patients in LTE who received rilzabrutinib monotherapy or with concomitant ITP medication. The number of patients in each group was fixed and did not change to monotherapy if patients discontinued concomitant ITP medication. ∗Day 85 may refer to visit day 85 or visit cycle 4, day 1, depending on duration of cycles. Day 169 may refer to visit day 169; cycle 1, day 1 LTE; or visit cycle 7, day 1, depending on the availability of patient’s visit information and duration of cycles. Platelet count threshold levels are denoted by the horizontal purple line at 30 × 109/L and the green line at 50 × 109/L. BID, twice daily.
Median platelet counts in patients who continued rilzabrutinib 400 mg BID in the LTE. (A) All 16 patients in LTE and (B) patients in LTE who received rilzabrutinib monotherapy or with concomitant ITP medication. The number of patients in each group was fixed and did not change to monotherapy if patients discontinued concomitant ITP medication. ∗Day 85 may refer to visit day 85 or visit cycle 4, day 1, depending on duration of cycles. Day 169 may refer to visit day 169; cycle 1, day 1 LTE; or visit cycle 7, day 1, depending on the availability of patient’s visit information and duration of cycles. Platelet count threshold levels are denoted by the horizontal purple line at 30 × 109/L and the green line at 50 × 109/L. BID, twice daily.
Of the 14 patients who received treatment with ≥1 monthly evaluation for >6 months on LTE, the median percentage of visits with a platelet response of ≥50 × 109/L was 92%. Among patients who had monthly or quarterly visits, ≥93% had a platelet response of ≥50 × 109/L for more than half of their monthly or quarterly assessments (supplemental Table 1).
Changing the dose is prohibited during the main treatment period except for safety reasons. Five of 11 (45%) patients receiving concomitant ITP therapy were able to stop using any concomitant ITP medication (CS, n = 2; TPO-RA, n = 1; CS and TPO-RA, n = 2) at a median of 254 days (range, 152-274) in the LTE period. At baseline, these 5 patients had a median age of 46 years (range, 35-61), and 3 (60%) were female. These patients had a median duration of ITP for 5.4 years (range, 1.8-7.8) and a median of 3 prior unique ITP therapies (range, 1-9), with only 1 patient who had a prior splenectomy. Their median platelet count at baseline of the main treatment period was 13 × 109/L (range, 4 × 109/L to 29 × 109/L). These patients had a median platelet count of 103 × 109/L (range, 90 × 109/L to 218 × 109/L) at the first platelet count measurement after stopping concomitant ITP medication and median platelet counts of 106 × 109/L (range, 75 × 109/L to 166 × 109/L) at 3 to 6 months after stopping concomitant ITP medication.
Safety
During the LTE, 13 patients (81%) had ≥1 any-cause AEs, with 3 patients (19%) experiencing grade ≥3 AEs. Treatment-related AEs occurred in 3 patients (19%) and all these events were grade 1 or 2 and transient; these included grade 2 upper respiratory tract infection, rhinorrhea, and vulvovaginal dryness and grade 1 cough and diarrhea (Table 4). Four patients (25%) experienced grade ≥2 infections: 1 patient with grade 2 treatment-related upper respiratory tract infection and 1 patient each with grade 2 bronchitis, grade 3 COVID-19, and grade 4 COVID-19 pneumonia, the latter all unrelated to treatment based on the independent investigator’s assessment. Three patients (19%) had serious AEs, which included grade 4 COVID-19 interstitial pneumonia on day 439 (interrupting rilzabrutinib for 7 days), grade 4 thrombocytopenia on day 386 (leading to rilzabrutinib discontinuation), and grade 3 pneumonia/grade 3 pulmonary embolism. The third patient had discontinued rilzabrutinib due to grade 3 worsening migraine on day 434 of rilzabrutinib treatment. This was followed 1 day later by grade 3 thrombocytosis, and grade 3 pneumonia/pulmonary embolism occurred 7 days after stopping rilzabrutinib during the safety follow-up; the patient was hospitalized to resolve the events and recovered. None of these events were deemed related to treatment by the investigator and all events resolved. There were no cases of anemia or neutropenia in the LTE. Liver function and electrocardiogram measurements were normal, with only a single occurrence of elevated alanine aminotransferase (3.4 times the upper limit of normal) on day 85 (day 1 of cycle 4) with no interruption of rilzabrutinib. There was no liver-related AE reported, and alanine aminotransferase was normalized in 14 days; this patient had a medical history of steatosis. The patient continued to receive rilzabrutinib up to day 318 of treatment. There were no occurrences of atrial fibrillation and no deaths in the LTE.
In the LTE, 6 patients (38%) had ≥1 bleeding event. According to investigator assessment, none of the 10 grade 1 events were deemed related to treatment. The bleeding events (with platelet counts before each event) included 2 events of epistaxis (69 × 109/L and 71 × 109/L) and 1 contusion (61 × 109/L) in the same patient, 2 events of gingival bleeding (34 × 109/L and 76 × 109/L) and 1 blood blister event (67 × 109/L) in the same patient, and 1 patient/event each with cerebral microhemorrhage (991 × 109/L), contusion (300 × 109/L; that is, left forearm bruise due to injury and not spontaneous), purpura (49 × 109/L), and traumatic hematoma (46 × 109/L). Further examination of the patient with cerebral microhemorrhage showed that the patient was diagnosed with COVID-19 15 days before and had not recovered at the time of the event. Rilzabrutinib was stopped 3 days before the cerebral microhemorrhage event, and the patient received aspirin (81 mg) 1 day before the cerebral microhemorrhage for migraine. The patient developed pneumonia due to COVID-19 and pulmonary embolism 4 days after the cerebral microhemorrhage. The event was independently deemed not related by the principal investigator.
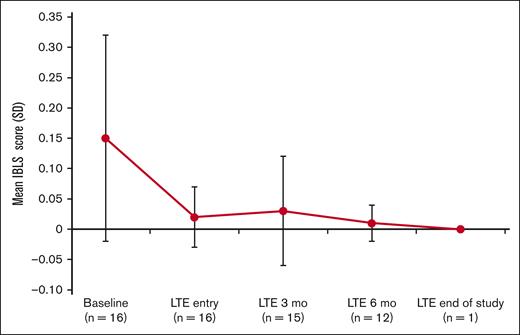
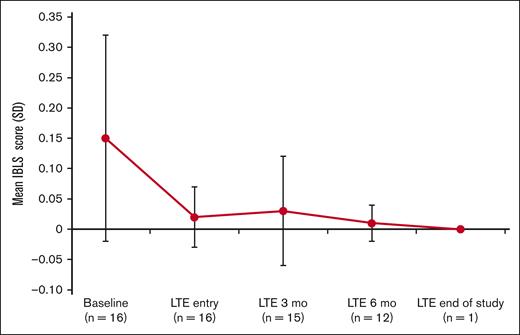
Assessment of the bleeding scale scores using the ITP Bleeding Scale-specific bleeding assessment tool indicated a decrease in the average bleeding score starting at 3 months in the LTE period (Figure 3).
IBLS bleeding scale scores at baseline, LTE entry (cycle 1, day 1), LTE 3 months (cycle 4, day 1), LTE 6 months (cycle 7, day 1), and LTE end of study. Bleeding symptoms were grouped by a total of 11 specific sites of bleeding and scored as 0, none; 1, 1 to 5 bruises and/or scattered petechiae; and 2, ≥5 bruises with size >2 cm and/or diffuse petechiae.29 The overall average (SD) score is calculated from the arithmetic mean of 11 site-specific grades. If ≥1 site was missing, the average of the nonmissing sites was used. IBLS, ITP Bleeding Scale.
IBLS bleeding scale scores at baseline, LTE entry (cycle 1, day 1), LTE 3 months (cycle 4, day 1), LTE 6 months (cycle 7, day 1), and LTE end of study. Bleeding symptoms were grouped by a total of 11 specific sites of bleeding and scored as 0, none; 1, 1 to 5 bruises and/or scattered petechiae; and 2, ≥5 bruises with size >2 cm and/or diffuse petechiae.29 The overall average (SD) score is calculated from the arithmetic mean of 11 site-specific grades. If ≥1 site was missing, the average of the nonmissing sites was used. IBLS, ITP Bleeding Scale.
QOL
Overall QOL scores using the EQ-5D VAS were measured monthly in the LTE. These results showed a baseline score for the main treatment period (before rilzabrutinib initiation) for the 16 patients in LTE with a mean of 84 (SD, 15). During this LTE, EQ-5D VAS scores were maintained at a high level on day 1 of each LTE monthly cycle 1, 3, 6, 9, and 12, with respective mean scores of 89 (SD, 10; n = 16), 90 (SD, 8; n = 16), 90 (SD, 9; n = 14), 88 (SD, 11; n = 12), and 89 (SD, 6; n = 8).
Discussion
The LTE phase of this global phase 1/2 clinical trial included 16 patients with ITP who previously had inadequate response to a median of 3 prior therapeutic options (range, 1-9; and including 3 patients [19%] with prior splenectomy) and who had achieved the primary end point during the initial main treatment period. In the main study, all had a rapid median time to a first platelet count of ≥50 × 109/L at 12.5 days; 8 of these patients had platelet counts of ≥50 × 109/L by day 8 (ie, early responders). With ongoing rilzabrutinib treatment at 400 mg twice daily in the LTE, platelet responses were sustained with a platelet count ≥50 × 109/L reported in 93% of patients for more than half of their monthly visits and maintained for a median of 88% of weeks on study. In the LTE, oral rilzabrutinib was well tolerated and associated only with grade 1 or 2 and transient treatment-related AEs.
The efficacy and safety results were consistent with the reported 24-week main treatment period in which 40% of the patients achieved a platelet response of ≥50 × 109/L and maintained this level for a mean of 65% of weeks during the 24-week treatment period, and rilzabrutinib treatment led to only low-grade AEs across all dose levels tested.23 Moreover, throughout the LTE, a high and durable platelet response with rilzabrutinib 400 mg twice daily was noted regardless of the use of concomitant ITP medication. Clinical benefit was maintained in patients who stopped using concomitant ITP medication but continued on rilzabrutinib. Importantly, rilzabrutinib was not associated with bleeding, arrhythmia, or other nonselective BTK inhibitor–associated AEs,30 and despite the very low rate of bleeding at baseline, bleeding scores improved even further with continued rilzabrutinib treatment in the LTE.
For any ITP therapy, it is important to assess the efficacy of and tolerability with prolonged exposure, along with individual patient needs and prior treatment history.31,32 Adequate platelet responses have been commonly observed with TPO-RAs, rituximab, fostamatinib, splenectomy, and immunosuppressive agents (eg, mycophenolate mofetil, vincristine, and cyclosporine).31,33,34 However, there is a high unmet need and notable lack of standard treatment for patients with ITP who are not responsive to such therapies. Long-term treatment with approved ITP therapies (romiplostim, eltrombopag, fostamatinib, and avatrombopag) has been evaluated in patients treated in phase 3 trials.35-38 In an open-label, extension study, 292 patients with ITP from combined phase 1 to 3 studies received up to 5 years of continuous weekly dosing with romiplostim.35 A platelet response of ≥50 × 109/L was achieved at least once at any time during the study in 95% of patients and maintained for a median of 92% of visits overall. Rescue medication was used in 33% of patients at least once during the study. Treatment-related AEs were documented in 35% of patients (8% serious AEs); by 24 weeks of treatment, nonserious headache was the only related AE with an incidence of 7%. Bleeding and thrombotic events were observed in 57% and 6.5% of patients, respectively. A similar LTE analysis was performed in the open-label, phase 3 EXTEND trial of patients who received the TPO-RA eltrombopag.36 Although 86% of patients had ≥1 platelet count of ≥50 × 109/L without the use of rescue medication, 32% of patients experienced grade ≥3 AEs (6% had a total of 24 thromboembolic events) and increased liver transaminases, pneumonia, anemia, and cataracts were among the most common grade ≥4 AEs or serious AEs associated with eltrombopag treatment. In the open-label, extension study with fostamatinib (FIT1 or FIT2 trials), although 44% of patients achieved the overall platelet response (defined differently as ≥1 platelet count of ≥50 × 109/L in the absence of rescue medication within 3 months of initiating fostamatinib), 75% of patients reported occurrence of AEs including 8% and 6% treatment-related severe AEs and serious AEs, respectively, with thrombocytopenia being the most frequently occurring in both cases.37 Extended avatrombopag therapy showed patients maintaining their initial response (platelet counts ≥50 × 109/L) for a mean of 83.3% of time on treatment during the core and extension phase before a loss of response to platelet counts <30 × 109/L on 2 consecutive scheduled visits.39 Although end points vary among studies, rilzabrutinib maintained and exceeded target levels of platelet counts over the majority of study weeks, with a favorable safety profile.
Each approved ITP treatment represents unique mechanisms of action to target ITP; romiplostim, eltrombopag, and avatrombopag are TPO-RAs that increase platelet production, whereas fostamatinib is a spleen tyrosine kinase inhibitor that reduces antibody-mediated platelet destruction.35-38,40 Although patients with ITP may achieve hemostatic platelet counts with many current therapies, a third of these patients still have early or chronic disease refractory to approved therapies.35-38,40 Along with differing stability and durability of platelet responses, their toxicity profiles differ widely, underscoring the need for novel therapeutic approaches that target the pathophysiological mechanisms of ITP, such as immune-mediated platelet destruction and impaired platelet production, while offering improved tolerability.
As a potent BTK inhibitor, rilzabrutinib provides a unique mechanism of action for treating patients with ITP. Rilzabrutinib mediates its therapeutic effects through multiple immune-mediated mechanisms including inhibition of B-cell activation and prevention of platelet phagocytosis by FcγR in spleen and liver, without altering platelet aggregation.20-22 The overall findings from the phase 1/2 main study and LTE provide preliminary evidence that rilzabrutinib could be a safe, well-tolerated, and effective treatment for patients with ITP who have failed other treatments. Unlike with TPO-RA,35,40 extreme elevations in platelet counts were not observed with rilzabrutinib treatment.
Limitations of this study include the open-label design of the trial, the lack of a control group, and the small sample size. Further investigations to evaluate the magnitude and durability of clinical benefit with rilzabrutinib are needed. The ongoing LUNA 3 randomized, double-blind, phase 3 trial was designed and is ongoing to compare rilzabrutinib with placebo in adults and adolescents (aged ≥12 years) with persistent or chronic ITP (NCT0456276641; EudraCT 2020-002063-6042).
Acknowledgments
The authors thank all patients, families, caregivers, and coinvestigators involved in this study. Statistical support and analyses were provided by Umer Khan and Mengjie Yao of Sanofi and Fiona Liu of SCiAN Services. Medical writing support was provided by Mihaela Marina and Julie Kern, Second City Science, LLC, and funded by Sanofi.
This study was supported by Sanofi.
Authorship
Contribution: D.J.K. designed the study; all authors (except A.D.) were study investigators; D.J.K. and A.D. analyzed results and generated the figures; and all authors reviewed and approved the submitted manuscript.
Conflict-of-interest disclosure: D.J.K. reports consultancy fees and research funding from Actelion (Syntimmune), Agios, Alnylam, Amgen, Argenx, BioCryst, Bristol Myers Squibb (BMS), Immunovant, Principia, Protalex, Rigel, Takeda (Bioverativ), and Union Chimique Belge (UCB); research funding from Kezar; and consultancy from Caremark, Cellphire, Cellularity, CRICO, Daiichi Sankyo, Dova, Genzyme, Hengrui, Incyte, Kyowa Kirin, Merck Sharp & Dohme, Momenta, Novartis, Pfizer, Platelet Biogenesis, Platelet Disorder Support Association, Sanofi, Shionogi, Shire, Up-To-Date, and Zafgen. J.M. received support and receipt of equipment, materials, drugs, medical writing, gifts or other services for current investigational study from Principia Biopharma (Sanofi). R. Baker reports research support/clinical trial funding for his institution from AbbVie, Acerta Pharma, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, CSL Behring, Daiichi Sankyo, Janssen-Cileg, Pfizer, Roche, Sanofi, Takeda, Technoclone, and Werfen. M.T. reports grants or contracts to the institution from Roche; consulting fees from Autolus, Caribou Biosciences, Genmab, Incyte, and Sobi; consulting fees and payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or education events from AbbVie, Amgen, BMS, Gilead Sciences, Janssen, MorphoSys, Novartis, Roche, and Takeda; and support for attending meetings and/or travel from AbbVie, BMS, Gilead Sciences, Janssen, Roche, and Takeda. P.Y.C. reports consultancy fees from Janssen Immunology, Nuvig, and Sobi, and speaker fees from Novartis. A.J.G.J. reports consultancy fees from Amgen and Novartis; speaker’s fees and travel cost payments from 3SBio, Amgen, and Novartis; international advisory board with Novartis; and research funding from Argenx, CSL Behring, Principia Biopharma (Sanofi), and Sobi. V.M. reports consultancy with Amgen, Argenx, Novartis, and Sobi; research funding from Grifols and Novartis; honoraria from Amgen, Argenx, Grifols, Novartis, and Sobi; and travel, accommodations, and expenses from Amgen. R. Bird reports being an investigator in clinical trials sponsored by Principia Biopharma (Sanofi) and Rigel, and serving on a speaker panel (nonremunerated) with Amgen. J.G. reports being an investigator in clinical trials sponsored by Principia Biopharma (Sanofi) and Rigel, and speaker fees from Amgen, Grifols, and Novartis. M.K. reports consultancy with Principia Biopharma Inc, a Sanofi company during the conduct of the study. T.G. reports research funding from Sobi; consulting for Cellphire and Sanofi; payment for lectures from Amgen and Sanofi; data safety and monitoring board for Palisade; honoraria from Sobi; and serving on advisory boards for Dova and Novartis. W.G. reports consultancy, honoraria, membership on an entity’s board of directors or advisory committees, travel and accommodations, and research funding from Alpine, Amgen, Argenx, Bayer, Grifols, Hutchmed, Kedrion, Novartis, Sanofi, Sobi, and UCB. A.D. reports current employment and is a current equity holder in publicly held company Sanofi. N.C. reports consultancy, honoraria, and research funding from Argenx, Grifols, Novartis, Principia, Rigel, Sanofi, Sobi, and UCB. The remaining authors declare no competing financial interests.
Correspondence: David J. Kuter, Hematology Division, Massachusetts General Hospital, Harvard Medical School, Bartlett Hall 150, 55 Fruit St, Boston, MA 02114-3908; email: kuter.david@mgh.harvard.edu.
References
Author notes
Presented in part as oral presentations at the American Society of Hematology 2020 (abstract 22) and 2021 (abstract 14), the European Hematology Association 2022 (abstract S291), and the International Society on Thrombosis and Hemostasis 2021 (abstract OC 72.2) and 2022 (abstract OC 73.1) annual meetings.
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and data set specifications. Patient level data will be anonymized, and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.vivli.org/.
The full-text version of this article contains a data supplement.