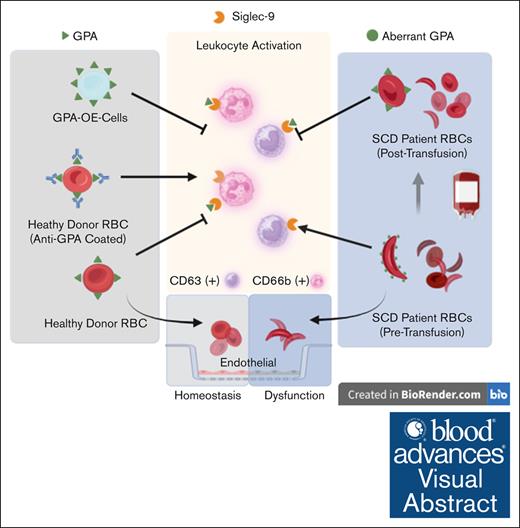
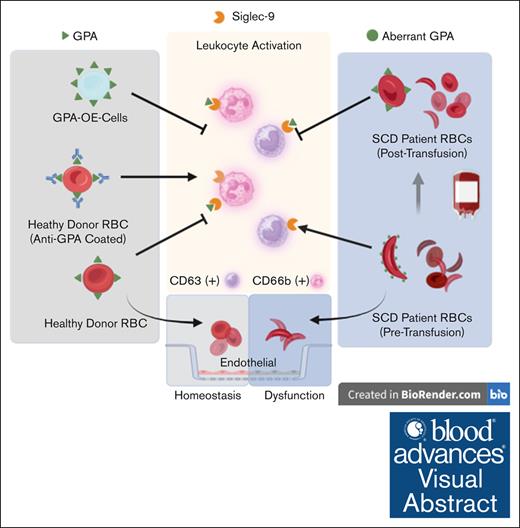
RBC transfusion can restore quiescence in previously activated immune cells under inflammatory conditions, such as sickle cell disease.
RBCs restore quiescence of activated leukocytes through their cell surface glycophorin A.
Visual Abstract
Glycophorin A (GPA), a red blood cell (RBC) surface glycoprotein, can maintain peripheral blood leukocyte quiescence through interaction with a sialic acid–binding Ig-like lectin (Siglec-9). Under inflammatory conditions such as sickle cell disease (SCD), the GPA of RBCs undergo structural changes that affect this interaction. Peripheral blood samples from patients with SCD before and after RBC transfusions were probed for neutrophil and monocyte activation markers and analyzed by fluorescence-activated cell sorting (FACS). RBCs were purified and tested by FACS for Siglec-9 binding and GPA expression, and incubated with cultured endothelial cells to evaluate their effect on barrier function. Activated leukocytes from healthy subjects (HS) were coincubated with healthy RBCs (RBCH), GPA-altered RBCs, or GPA-overexpressing (OE) cells and analyzed using FACS. Monocyte CD63 and neutrophil CD66b from patients with SCD at baseline were increased 47% and 27%, respectively, as compared with HS (P = .0017, P = .0162). After transfusion, these markers were suppressed by 22% and 17% (P = .0084, P = .0633). GPA expression in RBCSCD was 38% higher (P = .0291) with decreased Siglec-9 binding compared with RBCH (0.0266). Monocyte CD63 and neutrophil CD66b were suppressed after incubation with RBCH and GPA-OE cells, but not with GPA-altered RBCs. Endothelial barrier dysfunction after lipopolysaccharide challenge was restored fully with exposure to RBCH, but not with RBCSCD, from patients in pain crisis, or with RBCH with altered GPA. Pretransfusion RBCSCD do not effectively maintain the quiescence of leukocytes and endothelium, but quiescence is restored through RBC transfusion, likely by reestablished GPA-Siglec-9 interactions.
Introduction
Red blood cells (RBCs) have been extensively studied for their gas exchange capability in the lung and peripheral tissues. However, RBC transfusions also exert a wide array of immunomodulatory effects that either augment or suppress the immune system, a phenomenon known as transfusion-related immunomodulation.1 The transfusion-related immunomodulation effect was first reported when whole blood transfusions were observed to enhance graft survival after kidney transplantation.1 Although the chemical and morphological changes that occur in RBC units may lead to proinflammatory effects,2 there is evidence that RBC transfusion is immunosuppressive.3,4 Indeed, in vitro models suggest that anti-inflammatory interleukin-10 (IL-10) release and concomitant suppression of the proinflammatory tumor necrosis factor alpha response occur after coincubation of venous blood samples with leukoreduced allogeneic whole blood.3
The RBC is now recognized as a regulator of innate immunity.5 The RBC sialic acid moieties are primarily carried on the transmembrane sialoglycoprotein glycophorin-A (GPA), which interacts with a sialic acid–binding Ig-like lectin (Siglec-9) on the surface of peripheral blood leukocytes (ie, neutrophils and monocytes).6 The interaction between RBC surface GPA and lectin Siglec-9 maintains neutrophil and monocyte quiescence.7,8
The goal of this study was to further characterize the immunoregulatory properties of the RBC surface in ex vivo models of inflammation. Here, we focus on sickle cell disease (SCD) as an inflammatory condition in which RBCs may demonstrate structural alteration of the surface sialoglycoproteins that affect their interaction with immune cells. We hypothesize that RBCs from patients with SCD show decreased Siglec-9 binding and reduced immune modulatory effect on innate immune cell responders (ie, leukocytes and endothelial cells). We tested this hypothesis in a cohort of patients with SCD who were chronically transfused and evaluated the effect of RBC transfusion on their innate immune cell response.
Materials and methods
Study participants and blood collection
This study was approved by the University of Maryland Baltimore Institutional Review Board and conducted according to the Declaration of Helsinki. Patients at the University of Maryland Medical Center were included in the study based on the following criteria: (1) patients with sickle cell disease receiving chronic prophylactic RBC transfusions to prevent or treat vaso-occlusive crises and who (2) have no demonstrable history of RBC antibodies (see Table 1). Most subjects were stable (n = 19) and transfused prophylactically to prevent a vaso-occlusive crisis (VOC); 8 patients were in pain and transfused to treat VOC. Whole blood samples were drawn 20 minutes before transfusion and 20 minutes after transfusion for each subject for comparison of pre- and posttransfusion inflammatory markers. Leukocytes from whole blood pre- and posttransfusion samples were assessed for activation by flow cytometry (staining and analysis protocol described below). Healthy subjects (HS) with negative RBC antibody screens served as controls.
Red blood cell glycophorin A expression
Glycophorin A is an RBC surface glycoprotein that interacts with innate immune cells by binding to Siglec-9, an inhibitory sialic acid-binding Ig-like lectin. The level of GPA expression on the surface of RBCs from patients with SCD was assessed and compared with that of healthy subject RBCs. Whole blood samples were washed 5 times with 2.5 mM phosphate-buffered saline (PBS)-EDTA at 500 × g for 5 minutes. The RBC pellets were resuspended in magnetic-activated cell sorting (MACS) buffer and stained with phycoerythrin anti-GPA (Biolegend) for 30 minutes at 4°C in the dark, washed, and resuspended in MACS buffer for flow cytometry analysis.
Red blood cell Siglec-9 binding assay
Siglec-9 is the GPA-ligand on the surface of neutrophils and monocytes. Siglec-9 binding to RBCs from patients with SCD was assessed and compared with healthy subject RBCs. RBCs were purified as described above. RBC pellets were resuspended in PBS-EDTA and incubated with Fc-recombinant human Siglec-9 with an added Fc region (R&D Systems, Minneapolis, MN) (5 μg rSiglec-9/1 million RBCs) for 30 minutes at 4°C. Cells were then incubated with FITC anti-Fc (Millipore Sigma, Munich, Germany) and phycoerythrin anti-GPA (Biolegend, San Diego, CA), washed once, and resuspended in MACS buffer for flow cytometry analysis. Data are presented as mean fluorescence intensity (MFI).
Cell culture
Human pulmonary artery endothelial cells (HPAECs) and EGM-2 growth media kit were obtained from Lonza (Allendale, NJ) and cultured according to the manufacturer’s instructions. For the coculture experiments, HPAEC were used at early passages. Before the experiments, the EGM media was changed to basal media supplemented with 2% fetal bovine serum (FBS), unless otherwise specified.
TF-1 cells were cultured as described previously.9 Briefly, TF-1 cells, an erythroleukemia cell line, were obtained from ATCC (Manassas, VA) and genetically engineered to overexpress GPA. These cells were graciously donated to us by the Kingsbury Laboratory at the University of Maryland School of Medicine. Cells were cultured in RPMI medium (Gibco, ThermoFisher, Waltham, MA) containing 10% FBS and 1 ng/mL GM-CSF (Biolegend, San Diego, CA). Cells were split every 48 to 72 hours and plated at a density of 1 million cells per mL of medium. GPA overexpression was confirmed after fluorescent antibody staining and flow cytometry analysis, as described above, for RBC surface GPA.
Measurement of endothelial barrier function
Endothelial permeability was evaluated by monitoring the trans-endothelial electrical resistance (TER) across the HPAEC monolayers in an electric cell-substrate impedance sensing system (Applied Biophysics, Troy, NY). Briefly, cells reaching >1200 Ω of steady-state resistance were used for measurement of TER under experimental conditions, and normalized resistance values were plotted against time. HPAECs were incubated with RBCs from patients with SCD pre- and posttransfusion. Healthy subject RBCs served as controls. To investigate the effect of GPA on endothelial cell resistance, HPAECs were incubated with healthy RBCs pretreated with anti-GPA or neuraminidase as described below. To measure resistance to inflammatory damage induction, endothelial cells were first incubated with RBCs for 30 minutes and then challenged with LPS (200 ng/mL). TER was then measured continuously for up to 20 hours10 (data shown at 0, 5, 10, 15, and 20 hours).
Reversing leukocyte activation in vitro
As shown previously,7,8 whole blood-derived leukocytes are activated upon separation from RBCs. In light of the immunosuppressive properties of RBC transfusions in chronically transfused patients,11 we evaluated if leukocyte activation could be reversed in vitro by suspending them with aliquots of RBCs either coated or not coated with anti-GPA. Whole blood samples were centrifuged at 800 × g for 10 minutes. Buffy coats were then separated, resuspended in PBS containing 2% v/v FBS, and incubated with EasySep RBC Depletion Reagent (Stemcell Technologies, Vancouver, BC) in an EasySep magnet for removal of RBCs. Leukocytes were then separated from RBCs and as a result became activated as previously described.7 In order to test if their activation could be reversed, these were then washed and incubated at a 1:1000 leukocyte:RBC ratio with various RBC suspensions: (1) autologous healthy subject RBCs; as well as (2) autologous healthy subject RBCs either coated with anti-GPA (1:10) (clone JC159; Abcam, Cambridge, UK), or mouse IgG1 isotype serving as a control (Invitrogen, Carlsbad, CA); or (3) RBCs treated with sialidase (Clostridium perfringens neuraminidase (Sigma Aldrich, Munich, Germany) in PBS for 1 hour at 37°C, to cleave off sialic acid. In a separate suspension of leukocytes, in which activation had already been reversed by RBC incubation, GPA antibody at 1:10 dilution was added to disrupt the RBC-leukocyte inhibitory interaction, and mouse IgG1 isotype antibody (Invitrogen, Waltham, MA) served as a control.
TF-1 erythroleukemia cells engineered to overexpress GPA (GPA-OE) were cultured and tested to restore leukocyte quiescence. Leukocytes were coincubated with TF-1 cells at either 1:100 or 1:200 in a total volume of 100 μL of MACS buffer. Incubations were repeated in a transwell assay using 24-well plates (Corning, Corning, NY) containing 3 μM transwell inserts (Corning, Corning, NY). TF-1 cells were placed in the lower chamber, whereas leukocytes were placed in the upper chamber and removed for flow cytometry analysis.
Microvesicles (MVs) were purified from healthy subject RBC suspensions after incubation with the Piezo1 agonist, Yoda1 as previously described.12 Briefly, RBCs were stimulated with Yoda1 to trigger EV release, and then subjected to differential centrifugation to remove cell debris and apoptotic bodies. MVs were then isolated using centrifugation at 20 000 g for 1 hour at 4○C. GPA-positive MVs were identified using magnetic beads (Exosome immunoprecipitation kit (Protein G), Thermo Fisher Scientific, Reference number 10612D, Waltham, MA). These GPA-positive EVs were also tested to reverse leukocyte activation after incubation at a 1:20 000 leukocyte:MV ratio.
All leukocytes were incubated for 1 hour (for monocytes) or 24 hours (for neutrophils, whose activation is delayed compared with that of monocytes) at room temperature in the dark before being stained for viability and activation markers and analyzed by flow cytometry.
Fluorescent antibody staining
Before fluorescent antibody staining, all cells were incubated with Human TruStain FcX Fc Block (Biolegend, San Diego, CA) at a 1:50 ratio for 10 minutes at 4°C. For SCD and RBC coincubation experiments, cells were also incubated with 1:1000 fixable viability dye (Live/Dead Fixable Green; ThermoFisher, Waltham, MA) for 5 minutes at 4°C.
Samples incubated with RBCs and/or TF-1 cells were stained for CD45, CD41a, and either CD14 and CD63 (monocytes) or CD11b and CD66b (neutrophils) (all Biolegend, San Diego, CA). Samples incubated with TF-1 cells were stained for CD34 (Biolegend, San Diego, CA) to identify TF-1 cells. All the stained samples were incubated at 4°C for 30 minutes in the dark and washed with MACS buffer. Samples incubated with RBCs were resuspended in BD PhosFlow Lyse/Fix buffer at 4°C overnight, resuspended in MACS buffer the next day, and analyzed by flow cytometry. Samples incubated with TF-1 cells overexpressing GPA were similarly stained, except for the viability stain, which was performed using 7AAD viability dye (1:100) (Biolegend, San Diego, CA).TF-1 coincubation samples were not fixed and were analyzed on the same day.
Flow cytometry analysis
All samples, performed in triplicates, were acquired on a BD LSRII Fortessa cytometer using DIVA software and analyzed using FCS Express 7. Rainbow Calibration Particles (Biolegend, San Diego, CA) were used to standardize the cytometer settings between runs. Single-color compensation beads (Invitrogen, Carlsbad, CA) were used to calculate compensation. Samples incubated with RBCs were gated as previously described.8 For TF-1 experiments, the gating scheme was as follows: (1) FSC-A vs FSC-H to exclude doublets and large aggregates. (2) 7AAD vs FSC-H to exclude dead cells. (3) GFP (GPA Overexpression) or CD34 vs FSC-H to gate leukocytes and platelets to GFP/CD34null. (4) CD45 vs FSC-H to gate leukocytes to CD45high. From the leukocyte gate, neutrophils and monocytes were identified as follows: CD66b vs SSC-A to gate neutrophils as CD66bhigh and SSChigh and CD14 vs SSC-A to gate monocytes as CD14high and SSCmid.
All data on either monocyte or neutrophil activation from patients with SCD and HS are reported as relative median fluorescence intensity (rMFI), by normalizing the mean fluorescence intensity (MFI) of each triplicate value to the average MFI of the pretransfusion sample and then averaging the normalized triplicate values. Data on leukocyte activation after in vitro incubation with RBCs or GPA-OE TF-1 cells are reported as rMFI by normalizing the MFI of each triplicate value to the average MFI of the leukocyte-alone group. rMFI was used to eliminate the variation in MFI between patients and HS and to allow comparison of percent change between the treatment groups.
Data analysis
Normalization tests (Anderson-Darling test, D’Agostino & Pearson test, Shapiro-Wilk test, Kolmogorov-Smirnov test) were performed on all data sets using GraphPad Prism 8 and confirmed that the data were normally distributed. As sample sizes in all data sets were small, one-way analysis of variance (ANOVA) with Bonferroni post hoc test was used for comparison of leukocyte activation between patients with SCD and healthy control. For comparison of SCD leukocyte activation pre- and posttransfusion, paired t tests were used. For GPA expression and Siglec-9 binding experiments, student t tests were used. For all leukocyte plus RBC or TF-1 cell coincubations, one-way ANOVA with Bonferroni correction was used. For electrical cell-substrate impedance sensing analysis, resistance was taken at select time points and one-way ANOVA with Bonferroni correction were performed for each time point. For all tests, P ≤ .05 was considered statistically significant.
Results
Monocyte and neutrophil surface activation markers are suppressed in patients with SCD after RBC transfusion
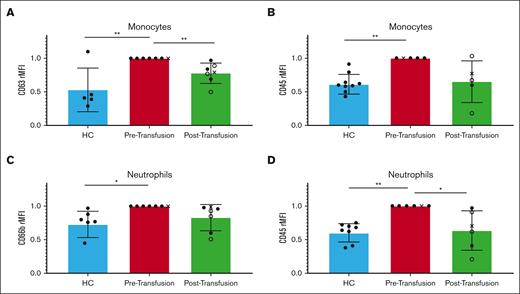
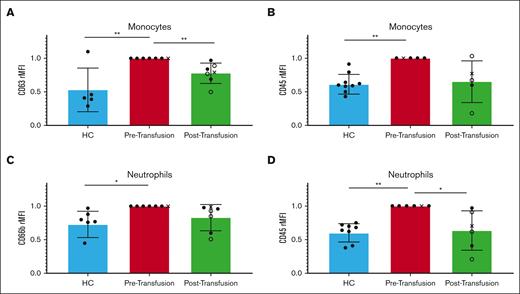
The expression levels of monocyte and neutrophil surface activation markers were first analyzed in peripheral blood samples drawn before transfusion from patients with SCD (n = 7) and compared with HS. These showed an increase (39% and 40%, respectively, P = .0038, P = .0015) in the surface expression of CD45, a tyrosine phosphatase, suggesting an increased threshold for activation compared with healthy individuals (Figure 1). Monocytes from patients with SCD showed a 47% increase (P = .0017) in CD63, a surface protein involved in membrane trafficking and signaling,13,14 whereas neutrophils showed a 27% increase (P = .0162) in CD66b, a granulocyte-specific surface activation marker involved in granule formation (Figure 1).15,16
Patients with sickle cell disease (SCD) present with activated leukocytes at baseline that are suppressed after RBC transfusion. The relative median fluorescence intensity (rMFI) of monocyte CD63 (A), CD45 (B), neutrophil CD66b (C), and CD45 (D) were measured in the peripheral blood of HS (blue) and compared with those from patients with SCD, pretransfusion (red), and posttransfusion (green) (n = 7). For the posttransfusion samples, filled circles represent patients receiving full RBC exchange, empty circles represent patients receiving partial RBC exchange, and X represents patients in active crisis. Healthy vs SCD leukocytes pretransfusion comparison done using one-way ANOVA with Bonferroni post hoc. SCD leukocytes pre- vs posttransfusion comparison using a paired t test. The error bars represent standard deviation. ∗ P < .05; ∗∗ P < .01.
Patients with sickle cell disease (SCD) present with activated leukocytes at baseline that are suppressed after RBC transfusion. The relative median fluorescence intensity (rMFI) of monocyte CD63 (A), CD45 (B), neutrophil CD66b (C), and CD45 (D) were measured in the peripheral blood of HS (blue) and compared with those from patients with SCD, pretransfusion (red), and posttransfusion (green) (n = 7). For the posttransfusion samples, filled circles represent patients receiving full RBC exchange, empty circles represent patients receiving partial RBC exchange, and X represents patients in active crisis. Healthy vs SCD leukocytes pretransfusion comparison done using one-way ANOVA with Bonferroni post hoc. SCD leukocytes pre- vs posttransfusion comparison using a paired t test. The error bars represent standard deviation. ∗ P < .05; ∗∗ P < .01.
Monocyte and neutrophil activation markers from patients with SCD were then analyzed after transfusion and compared with those before transfusion (n = 7). Monocyte CD63 expression decreased after transfusion by 22% (P = .0084), whereas CD45 decreased by 35% (P = .0646) as compared with that before transfusion. Neutrophil CD45 decreased significantly after transfusion by 36% (P = .0282), whereas CD66b decreased by 17% (P = .0633) compared with before transfusion (Figure 1).
GPA expression is increased on the surface of RBCs from patients with SCD, but Siglec-9 binding is decreased
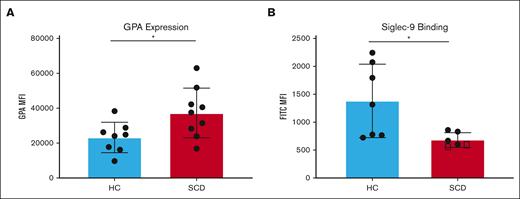
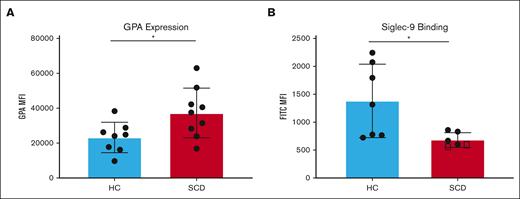
Leukocytes interact with RBC surface GPA through sialic acid-binding immunoglobulin-type lectin (Siglec) called Siglec-9.17 Siglec-9 is a sialic acid-binding immunoglobulin-type lectin present on immune cells that is known to suppress immune responses.7,18 The expression levels of RBC surface GPA, which maintains leukocyte quiescence in whole blood through Siglec-9 binding, were analyzed in purified RBCs from patients with SCD and HS (n = 9). RBCs from patients with SCD showed a 37.8% higher level of GPA expression than RBCs from HS (P = .0291) (Figure 2A).
RBCs from patients with SCD present higher surface GPA expression with reduced Siglec-9-Fc binding. The median fluorescence intensity (MFI) of surface GPA was measured on healthy RBCs (blue) and on RBCs of patients with SCD pretransfusion (red) (n = 9) (A). The MFI for Siglec-9-Fc binding (B) was measured in RBC from healthy RBCs (blue), RBCs of patients with SCD pretransfusion (n = 4) (red), and from patients with untransfused SCD with >90% HbS (n = 2) (marked with squares); comparison performed using unpaired t tests. Error bars represent standard deviation. ∗ P < .05.
RBCs from patients with SCD present higher surface GPA expression with reduced Siglec-9-Fc binding. The median fluorescence intensity (MFI) of surface GPA was measured on healthy RBCs (blue) and on RBCs of patients with SCD pretransfusion (red) (n = 9) (A). The MFI for Siglec-9-Fc binding (B) was measured in RBC from healthy RBCs (blue), RBCs of patients with SCD pretransfusion (n = 4) (red), and from patients with untransfused SCD with >90% HbS (n = 2) (marked with squares); comparison performed using unpaired t tests. Error bars represent standard deviation. ∗ P < .05.
RBCs were incubated with recombinant Siglec-9 protein and analyzed by flow cytometry to detect binding levels to RBCs. RBCs from patients with SCD showed a 31.5% decrease in Siglec-9 binding compared with RBCs from HS (P = .2125) (Figure 2B). The decrease in Siglec-9 binding to RBCs from patients with SCD may disrupt leukocyte quiescence and is consistent with data above showing increased monocyte and neutrophil activation in SCD whole blood. Importantly, with the inclusion of 2 patients with SCD who were not chronically transfused and for whom hemoglobin S level was >95%, Siglec-9 binding was reduced by 50% compared with RBCs from HS (P = .0266) (Figure 2B). Siglec-9 binding and HPLC quantification of HbS levels in healthy subjects, patients with SCD with low HbS, and patients with SCD with high HbS are shown in supplemental Figure 1 and supplemental Figure 2. The decrease in Siglec-9 binding to RBCs from patients with SCD may disrupt leukocyte quiescence and is consistent with data above showing increased monocyte and neutrophil activation in SCD whole blood.
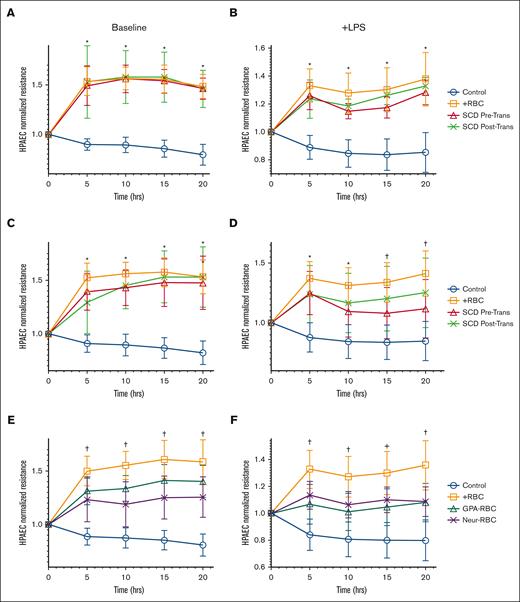
RBCs from patients with SCD in crisis reduce endothelial cell barrier function, similar to RBCs with altered GPA
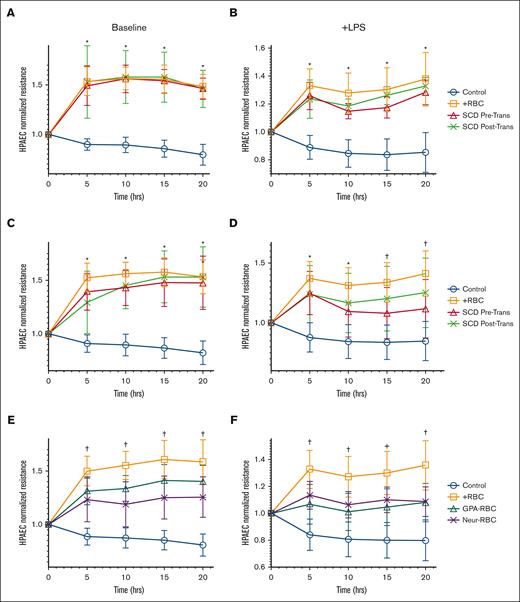
Previous data have suggested that RBCs promote endothelial barrier integrity and attenuate injury in response to inflammation.10 We measured trans-endothelial resistance (TER) across a confluent layer of HPAECs after incubation with purified RBCs from stable patients with SCD (not in pain) obtained before and after transfusion or RBCs from HS (n = 6). HPAEC incubation with RBCs from HS increased TER by 1.5-fold (P < .0001) compared with endothelial cells alone, similar to the increase in HPAEC TER when incubated with RBCs obtained from stable patients with SCD pre-and posttransfusion increase (P < .0001) (Figure 3A). Importantly, the supernatant from the endothelial cell (HPAEC) culture ECIS was analyzed and was negative for the presence of either free heme or hemoglobin, which could have activated HPAECs after a 20-hour incubation of RBCs (data not shown).19
RBCs from patients with SCD and RBCs with altered GPA have a reduced protective effect on the endothelium. Transendothelial electrical resistance (TER) is represented at different time points over 20 hours, for human pulmonary arterial endothelial cells (HPAECs) exposed at baseline or with LPS challenge, to RBCs (1:20) from stable patients with SCD (no pain; n = 6) (A-B); from patients with SCD in pain crises (n = 8) (C-D); and from HS but treated with anti-GPA or with neuraminidase to alter GPA on the RBC surface (E-F). TER was measured with incubation of RBC samples drawn from patients either before transfusion (red triangles) or after transfusion (green Xs) (A-D), and on HS’ RBC samples treated with anti-GPA (teal triangles) or treated with neuraminidase (purple Xs) (E-F). Multiple comparisons at each time point were made between groups using one-way ANOVA with Bonferroni post hoc for statistical analysis. Significance is marked as ∗ P < .05 for comparison with HPAEC alone (blue); or as † P < .05 for comparisons with HPAEC incubated with HS’ RBCs (orange). LPS (200 ng/mL) challenge was performed at 30’ min after start of RBC incubations.
RBCs from patients with SCD and RBCs with altered GPA have a reduced protective effect on the endothelium. Transendothelial electrical resistance (TER) is represented at different time points over 20 hours, for human pulmonary arterial endothelial cells (HPAECs) exposed at baseline or with LPS challenge, to RBCs (1:20) from stable patients with SCD (no pain; n = 6) (A-B); from patients with SCD in pain crises (n = 8) (C-D); and from HS but treated with anti-GPA or with neuraminidase to alter GPA on the RBC surface (E-F). TER was measured with incubation of RBC samples drawn from patients either before transfusion (red triangles) or after transfusion (green Xs) (A-D), and on HS’ RBC samples treated with anti-GPA (teal triangles) or treated with neuraminidase (purple Xs) (E-F). Multiple comparisons at each time point were made between groups using one-way ANOVA with Bonferroni post hoc for statistical analysis. Significance is marked as ∗ P < .05 for comparison with HPAEC alone (blue); or as † P < .05 for comparisons with HPAEC incubated with HS’ RBCs (orange). LPS (200 ng/mL) challenge was performed at 30’ min after start of RBC incubations.
To model the chronic vascular inflammation found in patients with SCD, RBC samples were tested to improve the endothelial barrier challenged with LPS. In this model, RBCs from HS restored TER to 1.4-fold greater than endothelial cells alone challenged with LPS alone (P < .0001). RBCs sampled before and after transfusion from stable patients with SCD (with no pain, n = 6) restored TER by 1.2- and 1.5-fold, respectively, compared with baseline endothelial cells challenged with LPS (P = .007 and P < .0001), consistent with a sustained posttransfusion protective effect on the endothelium (Figure 3B). RBCs sampled before and after transfusion from patients with SCD in VOC with pain (n = 8) also increased TER significantly compared with HPAEC alone (Figure 3C). However, when the HPAECs were challenged with LPS, TER was not significantly restored (1.1-fold; P = .1217) with pretransfusion RBCs compared with HPAEC alone. After transfusion, the sampled RBCs raised the TER significantly to 1.4 times as compared with baseline HPAECS alone after LPS challenge (P = .0028) (Figure 3D), suggesting that transfusion of healthy RBCs may improve endothelial barrier protection in patients with SCD in a pain crisis.
Considering the altered expression of GPA in RBCs from patients with SCD, suggesting that GPA integrity may play a role in endothelial barrier protection against inflammation, TER was measured across HPAECs incubated with RBCs pretreated with either anti-GPA (n = 18) or neuraminidase (n = 7), both at baseline and after LPS challenge. At baseline (without LPS challenge), GPA antibody treated RBCs increased in TER to 1.4 times and neuraminidase-RBCs increased TER by 1.2 times compared with HPAEC alone (Figure 3E). Importantly, the increase in TER observed by GPA altered RBCs was significantly less than that observed in healthy RBCs from TER of HPAEC alone (P < .05). Furthermore, after inflammatory LPS challenge, GPA antibody- and neuraminidase-treated RBCs increased TER by 1.1 times, significantly less than the increase in healthy RBCs (1.3 times) from HPAEC alone challenged with LPS (P < .05) (Figure 3F).
Leukocyte activation is suppressed after incubation with healthy RBCs but not with anti-GPA-treated or neuraminidase-treated RBCs
To assess whether the surface expression of monocyte CD63 and neutrophil CD66b observed in patients with SCD at baseline may be due to altered GPA in SCD RBCs, we first tested RBC samples from HS, treated them with either anti-GPA or neuraminidase, and incubated them with activated monocytes and neutrophils. Monocytes and neutrophils were activated by simple separation from whole blood as previously described by Lizcano et al.7
Monocytes were then probed for the surface expression of CD63, also known as LAMP-3, which plays a role in cellular signaling cascades20 and immune stimulation.14-16 CD63 expression from isolated monocytes separated from whole blood served as the reference activated control group, and we have previously shown that CD63 is significantly increased in isolated monocytes from buffy coats compared with monocytes in whole blood.8 CD63 expression in isolated monocytes was suppressed by 26% after incubation with autologous RBCs (n = 7) at a 1000:1 ratio (P = .0464) (Figure 4A).
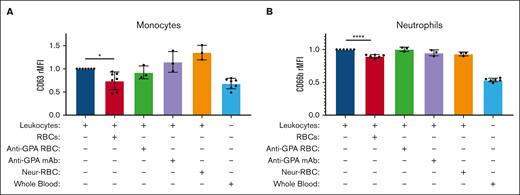
Monocyte and neutrophil activations are suppressed after incubation with healthy RBCs but not with neuraminidase-treated RBCs on their surface. The relative median fluorescence intensities (rMFIs) of CD63 (A) and CD66b (B) were measured on monocytes and neutrophils activated at baseline from the separation of whole blood samples from healthy donors (dark blue), and then coincubated with either healthy RBCs (red), anti-GPA-treated RBCs (green), healthy RBCs and indirectly with anti-GPA (purple), and neuraminidase-treated RBCs (orange). Leukocytes not separated from healthy donor whole blood (light blue) are shown as baseline (all n > 3). A one-way ANOVA test with Bonferroni post hoc was performed. ∗ P < .05; ∗∗∗∗ P < .0001.
Monocyte and neutrophil activations are suppressed after incubation with healthy RBCs but not with neuraminidase-treated RBCs on their surface. The relative median fluorescence intensities (rMFIs) of CD63 (A) and CD66b (B) were measured on monocytes and neutrophils activated at baseline from the separation of whole blood samples from healthy donors (dark blue), and then coincubated with either healthy RBCs (red), anti-GPA-treated RBCs (green), healthy RBCs and indirectly with anti-GPA (purple), and neuraminidase-treated RBCs (orange). Leukocytes not separated from healthy donor whole blood (light blue) are shown as baseline (all n > 3). A one-way ANOVA test with Bonferroni post hoc was performed. ∗ P < .05; ∗∗∗∗ P < .0001.
Neutrophils were probed for CD66b expression (n = 7), secondary degranulation, and surface activation marker.16 Similar to the isolated monocytes described above, isolated neutrophils from whole blood served as the reference activated control group. CD66b expression in isolated neutrophils was also suppressed by 10.2% after incubation with autologous RBCs at a 1000:1 ratio (P = .0388) (Figure 4B).
To test whether GPA plays a role in the RBC-mediated immunoregulatory effect, suppression of monocyte and neutrophil activations were tested after incubation with RBCs coated with GPA antibodies (n = 3). Neither monocyte CD63 nor neutrophil CD66b expression was significantly suppressed after coincubation with anti-GPA-coated RBCs, which disrupted the GPA-Siglec-9 interaction (n = 3) (P > .99). Furthermore, indirect GPA blockade by adding anti-GPA separately after healthy RBC incubation did not suppress monocyte CD63 or neutrophil CD66b (Figure 4AB).
To further refine the effect of GPA alteration on the activation level of monocytes and neutrophils, RBCs were treated with neuraminidase, a sialidase that removes sialic acid from the surface of RBC GPA (n = 3). Monocyte CD63 expression and neutrophil CD66b expression were not suppressed after incubation with neuraminidase-treated RBCs compared with isolated respective controls (Figure 4A-B).
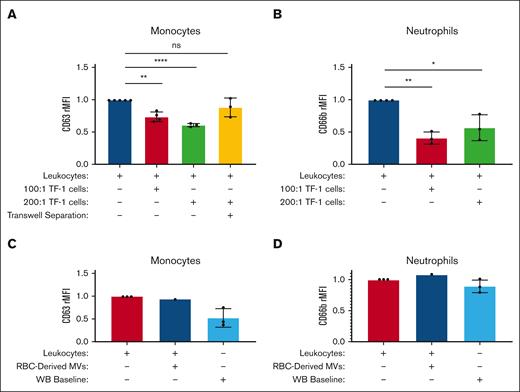
Monocyte CD63 and neutrophil CD66b expression were suppressed upon incubation with TF-1 cell lines overexpressing (OE) GPA
After a 1-hour incubation with GPA-OE TF-1 cells (n = 4), monocyte CD63 expression decreased by 26.5% (when incubated at 100:1 for TF-1:leukocyte titer) and by 39.3% when incubated at 200:1 compared with isolated activated monocytes alone (P = .0004 and P < .0001).
After a 24-hour-incubation with GPA-OE TF-1 cells (n = 3), neutrophil CD66b expression was decreased by 59.3% when incubated at 100:1 and by 43.3% when incubated at a 200:1 titer compared with isolated activated neutrophils alone (P = .0017 and P = .0168) (Figure 5A).
Monocyte and neutrophil surface activation marker expression is suppressed after incubation with GPA-overexpressing TF-1 cells in a contact- and dose-dependent manner. The relative median fluorescence intensities (rMFIs) of monocyte CD63 (n = 4) (A) and neutrophil CD66b (B) (n = 3) were measured for isolated activated leukocytes from healthy donors alone (blue), or coincubated with GPA Overexpressing TF-1 cells at either a 100:1 ratio (red), or at a 200:1 ratio (green), and incubated with GPA Overexpression TF-1 cells (200:1) separated by a transwell insert (yellow). rMFI of monocyte CD63 (C) and neutrophil CD66b (D) for isolated leukocytes alone (red) or after incubation with healthy RBC-derived GPA-microvesicles at 20 000:1 ratio (dark blue), with leukocytes in whole blood serving as a negative control baseline (light blue). A one-way ANOVA test with Bonferroni post hoc was performed. ∗ P < .05; ∗∗ P < .01; ∗∗∗∗ P < .0001; ns P > .05.
Monocyte and neutrophil surface activation marker expression is suppressed after incubation with GPA-overexpressing TF-1 cells in a contact- and dose-dependent manner. The relative median fluorescence intensities (rMFIs) of monocyte CD63 (n = 4) (A) and neutrophil CD66b (B) (n = 3) were measured for isolated activated leukocytes from healthy donors alone (blue), or coincubated with GPA Overexpressing TF-1 cells at either a 100:1 ratio (red), or at a 200:1 ratio (green), and incubated with GPA Overexpression TF-1 cells (200:1) separated by a transwell insert (yellow). rMFI of monocyte CD63 (C) and neutrophil CD66b (D) for isolated leukocytes alone (red) or after incubation with healthy RBC-derived GPA-microvesicles at 20 000:1 ratio (dark blue), with leukocytes in whole blood serving as a negative control baseline (light blue). A one-way ANOVA test with Bonferroni post hoc was performed. ∗ P < .05; ∗∗ P < .01; ∗∗∗∗ P < .0001; ns P > .05.
To test whether direct contact was required for GPA to maintain leukocyte quiescence, monocytes and GPA-OE TF1 cells were separated using a transwell. Monocytes incubated with GPA-OE TF-1 cells, but separated by a transwell insert, were not suppressed compared with isolated monocytes incubated alone (P > .9999 for both) (Figure 5A). Similarly, neutrophil activation was not suppressed (data not shown), suggesting that cell contact was required for GPA-mediated suppression.
GPA-overexpressing microvesicles did not reduce leukocyte activation
Activated leukocytes were incubated with the MVs derived from HS’ RBCs. Neither monocyte CD63 expression nor neutrophil CD66b expression was suppressed compared with isolated activated leukocytes alone (Figure 5B).
Discussion
RBCs are increasingly being recognized as modulators of innate immunity.5 In this study, a chronic inflammatory state was observed at the baseline in a cohort of patients with SCD. Indeed, SCD peripheral blood monocytes and neutrophils are poised towards activation with increased monocyte CD63 and neutrophil CD66b surface expression compared with HS. Importantly, after RBC transfusion, the expression of these activation surface markers was suppressed, and approximated baseline quiescence.
The current study by our group confirms the immunomodulatory effects of RBC transfusion on the inflammatory state observed in patients with SCD. However, in a previous study by Dembele et al on chronically transfused patients with SCD, the level of neutrophil activation measured before transfusion was not significantly suppressed compared with patients with SCD maintained on hydroxyurea therapy without transfusion,21 suggesting that chronic activation of neutrophils is characteristic of SCD, as healthy RBCs move out of circulation and are replaced by the patient’s SCD RBCs. These data are in line with our results, confirming that the chronic inflammatory state is a hallmark of sickle cell disease.22,23 Nevertheless, our study as well as Boyle et al.24
This study extends a previous observation by our group showing that under inflammatory stress, the endothelial barrier is protected when in contact with RBCs from HS.10 Indeed, RBCs from patients with SCD obtained before and after transfusion demonstrated different effects in our endothelial assay, depending on whether the patients were stable or in pain crisis. RBCs obtained from patients in a pain crisis before transfusion did not improve endothelial barrier function challenged with LPS, whereas RBCs obtained after transfusion did convey an improvement in endothelial function. Conversely, the inability of RBCs from patients with SCD to mitigate endothelial dysfunction due to LPS challenge is in line with the chronic vascular inflammation known to be a hallmark of SCD, which contributes to the development of vaso-occlusive pain crises.25 These results are consistent with those of a study by Hyacinth et al, which showed a decrease in circulating endothelial inflammatory markers in patients with SCD after transfusion.26 Furthermore, our data agree with observations from a Brazilian cohort study of patients with SCD who demonstrated a reduced risk of vaso-occlusive crises with chronic transfusion therapy.27 It is hypothesized that endothelial activation is one of the main drivers of chronic pain in patients with SCD,28 and mitigating endothelial activation in patients from SCD through transfusion may help reduce the pain these patients experience.
We and others have demonstrated that GPA, a sialoglycoprotein on the RBC surface, maintains leukocyte quiescence in the peripheral blood.7,8 Importantly, GPA expression on the surface of RBCs from these patients with SCD was increased at baseline compared with HS’ RBCs, but showed decreased binding to recombinant Siglec-9, a lectin leukocyte surface ligand for GPA that maintains leukocyte quiescence. Ashwood et al explored the glycan expression profile of RBCs in patients with SCD and also showed variable changes of sialylation with increased α2,6 linkages (ie, GPA) compared with healthy donor RBCs.29 These findings are also consistent with those of Kiser et al, who showed that RBCs from patients with SCD had decreased Siglec-9 binding, whereas the availability of sialic acid on the RBC surface was increased compared with healthy control RBCs.30,31 However, data from Aminoff and Ekeke showed decreased GPA/sialic acid content in RBCs from patients with SCD compared with HS.32,33 Thus, further investigations of the structural alterations of GPA and/or its sialic acid surface content are required to determine the RBC ability to interact with immune cells.
In vitro analysis of the effect of various alterations in GPA and its sialic acid showed that RBCs treated with either neutralizing GPA antibody or neuraminidase, which both decreased Siglec-9 binding, lost their ability to restore leukocyte quiescence as well as endothelial barrier protection. Siglec-9 is not expressed on HPAEC (data not shown); thus, it is likely that a different Siglec on endothelial cells interacts with GPA. Nevertheless, these findings strongly suggest that restoration of immune cell quiescence is RBC-GPA- and sialic acid-dependent. Sialic acid glycosylation is found on nearly all cells in the body and plays an important role in modulating both innate and adaptive immune responses.18,34 Importantly, this role often involves binding to Siglecs, a family of receptors typically linked to an inhibitory signaling motif.17 In support of these data, monocyte and neutrophil quiescence were restored upon coincubation with GPA-OE cells. This effect could not be reproduced in a transwell assay, suggesting that cell contact between leukocytes and GPA-OE cells is required.
The role played by GPA-expressing microvesicles in modulating the activation state of leukocytes was also investigated. RBC-derived microvesicles expressing GPA on their surface may inhibit macrophage cytokine production35 and inhibit B cell differentiation and antibody production in vitro.36 Paradoxically, Danesh et al found that in vitro coincubation of monocytes with RBC-derived exosomes increased proinflammatory cytokine production.37 However, our findings are consistent with those of Muszynski et al, who showed no effect of RBC-derived microvesicles on monocyte or neutrophil activation.4 These paradoxical results can be explained by the highly heterogeneous composition of microvesicles and the environment in which they are generated.38
One major limitation of the study is that most of the RBC samples analyzed were not pure sickle RBC fractions but whole blood samples from patients who received regular RBC transfusions. The average HbS level across the patient samples studied was 35% before transfusion and <20% after transfusion. Nevertheless, the decrease in leukocyte activation after transfusion was consistent with the dilution of the sickle cell effect in peripheral blood. Another limitation is that the ECIS model of endothelial barrier function is static, and thus may not provide detailed information as a microfluidic model or in vivo system, in which RBCs are free flowing with other cell types included.
In conclusion, this study confirms that patients with SCD present with a proinflammatory state of their immune cells and unveils a beneficial role of RBC transfusion in restoring their quiescence and in protecting the endothelial barrier against inflammation. This immunomodulatory function of RBCs is mediated by GPA, a sialylated-Siglec ligand, which is altered in patients with SCD. As such, future studies will focus on the structural analysis of RBC GPA and its Siglec-interactions in SCD.
Acknowledgments
The authors acknowledge the support from the Maryland Stem Cell Research Foundation Funds (2020-MSCRFD-5388 MSCRF Discovery Program). The authors acknowledge the support of the University of Maryland, Baltimore, Institute for Clinical & Translational Research (ICTR) and the National Center for Advancing Translational Sciences (NCATS) Clinical Translational Science Award (CTSA) grant number 1UL1TR003098. The authors acknowledge the support from the National Institutes of Health funds for the Buehler Laboratory – (R01 HL161004 and R01 HL158076) and for the Birukov laboratory (HL076259 and HL146829), as well as the National Institutes of Health, United States (grant IOS-2235553).
Authorship
Contribution: M.J.F. and G.R.V. designed the experiments and supervised their execution; J.N.M. and M.J.F. analyzed the data and wrote the manuscript; J.N.M., M.N.K., P.K., K.P., and S.S. performed the experiments; X.F. advised reviewing all FACS gating and analyses; and K.G.B. and P.W.B. provided secondary reviews of the analyses, presentation of the data, and editing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Magali Fontaine, Department of Pathology, University of Maryland School of Medicine, Director of Transfusion Services, 22 S Greene St N2W50a, Baltimore, MD 21201; email: mfontaine@som.umaryland.edu.
References
Author notes
Any renewable materials, data sets, and protocols will be made available upon request by email to the corresponding author Magali Fontaine (mfontaine@som.umaryland.edu). Original data are deposited in a repository and can be accessed upon request to the corresponding author Magali Fontaine (mfontaine@som.umaryland.edu).
The full-text version of this article contains a data supplement.