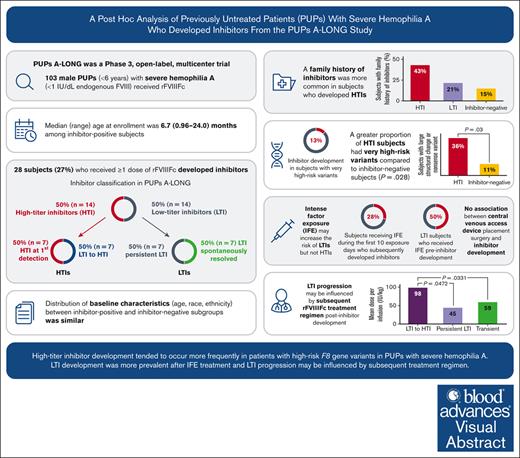
This report provides a post hoc analysis of males with severe hemophilia A who developed inhibitors in the PUPs A-LONG trial.
High-risk F8 variants and factor dosing were associated with high-titer inhibitors (HTI) and low-titer to HTI progression, respectively.
Visual Abstract
Inhibitor development is a major therapeutic complication for people with hemophilia. The phase 3 PUPs A-LONG study evaluated the safety and efficacy of efmoroctocog alfa (a recombinant factor VIII Fc fusion protein, herein referred to as rFVIIIFc) in previously untreated patients (PUPs) with severe hemophilia A. Male PUPs <6 years old were enrolled and received rFVIIIFc; inhibitor development was the primary end point. Post hoc analyses, including patient treatment regimen patterns and timing of inhibitor development, descriptive and Kaplan-Meier analyses of time to first inhibitor-positive test by treatment regimen and by titer, and consumption, were performed to describe patients who developed inhibitors during PUPs A-LONG. We investigated patient characteristics (eg, demographics and genotype) and nongenetic risk factors (eg, intense factor exposure and central venous access device [CVAD] placement) that may predict inhibitor development and characteristics of inhibitor development (low-titer vs high-titer inhibitor). Baseline characteristics were similarly distributed for age, race, and ethnicity across both patients who were inhibitor-positive and those who were inhibitor-negative (all P > .05). High-risk F8 variants were associated with development of high-titer inhibitors (P = .028). High-titer inhibitor development was often preceded by the presence of a low-titer inhibitor. Patients whose low-titer inhibitor progressed to a high-titer inhibitor received a higher mean dose per infusion (98.4 IU/kg, n = 5) compared with those whose low-titer inhibitor resolved spontaneously (59.2 IU/kg, n = 7; P = .033) or persisted (45.0 IU/kg, n = 5; P = .047). There was no association between CVAD placement surgery and inhibitor development. Post hoc analyses suggest that F8 genotype and dose of factor are as important as inhibitor risk factors and require further investigation. This study was registered at ClinicalTrials.gov as #NCT02234323.
Introduction
Severe hemophilia A is an inherited bleeding disorder characterized by spontaneous bleeds and prolonged bleeding after trauma and/or surgery. It is caused by a deficiency of functional factor VIII (FVIII), with symptoms typically beginning in early childhood.1 Prophylactic FVIII replacement therapy is the standard of care in people with severe hemophilia A. Frequent intravenous infusions with traditional FVIII concentrates for prophylaxis or on-demand treatment often necessitate the insertion of central venous access devices (CVADs), especially in young children.1,2 Extended half-life FVIII products aim to reduce the frequency of infusions, lessen the need for CVADs, and improve treatment adherence.3
Despite advances in hemophilia care, inhibitor development remains a significant challenge for hemophilia management, considering that inhibitors (neutralizing alloantibodies) render FVIII replacement ineffective for treatment or prevention of bleeds.1 Inhibitor development occurs in 25% to 40% of previously untreated patients (PUPs) with severe hemophilia A exposed to standard half-life FVIII concentrates.4-9 Inhibitors tend to develop within the first 20 exposure days (EDs) to FVIII among people with severe hemophilia A, although inhibitor development has been documented up to 75 EDs.10
Inhibitors are high or low titer depending on peak titer value detected by Bethesda assay and further classified as high- or low-responding inhibitors based on presence (high responding) or absence (low responding) of an anamnestic response.1,11 High- and low-titer inhibitors require different management approaches because high-titer inhibitors (HTIs) tend to be persistent and make the use of replacement FVIII concentrates for prophylaxis and for acute bleed treatment no longer effective, whereas low-titer inhibitors (LTIs) can be transient, although a significant proportion have been shown to subsequently convert to HTIs.1,12 Inhibitor development is multifactorial13; several nonmodifiable genetic risk factors have been associated with the development of inhibitors, including F8 gene mutation, a family history of inhibitors, and ethnicity.14,15 Nongenetic factors that may influence the development of inhibitors include environmental factors (eg, infection, surgery, and trauma) and treatment-related factors (ie, dosing regimen, intense factor exposure [IFE], and type of factor product).6,16-18
Efmoroctocog alfa (a recombinant FVIII Fc fusion protein, herein referred to as rFVIIIFc; Eloctate and Elocta, Sanofi, Waltham, MA and Sobi, Stockholm, Sweden) is an extended half-life FVIII replacement therapy approved for routine prophylaxis, on-demand treatment, and perioperative management of bleeding in people of all ages with hemophilia A. Long-term safety and efficacy have been demonstrated for previously treated adults and children with severe hemophilia A in the A-LONG study (NCT01181128),19 kids A-LONG study (NCT01458106),20 and the ASPIRE extension study (NCT01454739).21
The PUPs A-LONG study (NCT02234323) evaluated safety and efficacy of rFVIIIFc in PUPs with severe hemophilia A, with inhibitor development being the primary end point.22 Overall incidence of inhibitor development was within the expected range (31.1% [95% confidence interval (CI), 21.8-41.7] among patients with ≥10 EDs or among those who developed an inhibitor irrespective of when the inhibitor occurred; 27.7% [95% CI, 19.3-37.5] among all patients, regardless of number of EDs; this includes patients who had not received ≥10 EDs to rFVIIIFc). rFVIIIFc was well tolerated and effective for prophylaxis and bleeding control. Incidence of HTIs was 15.6% (n = 14; 95% CI, 8.8-24.7).22
Considering that factors influencing inhibitor development are not fully understood at individual patient level, we performed a series of post hoc analyses to provide a description of patients who developed inhibitors during the PUPs A-LONG study.
Methods
Study design and population
Full study details of the phase 3, open-label, multicenter PUPs A-LONG trial have been reported previously.22 Briefly, males <6 years of age with severe hemophilia A (˂1 IU/dL endogenous FVIII [1%]) and no prior exposure to any blood components or any FVIII concentrates, were eligible for study inclusion and received rFVIIIFc treatment. The treatment period was ≥50 EDs to rFVIIIFc, unless early withdrawal occurred, or end of study was declared. Investigators had the option to treat a patient on demand; however, according to global standards of care, prophylaxis was recommended before or immediately after a third joint bleed.
The primary end point of PUPs A-LONG was the occurrence of inhibitor development assessed by the Nijmegen-modified Bethesda assay (defined as ≥0.60 BU [Bethesda units]/mL, confirmed by a second positive inhibitor test 2 to 4 weeks after the original sample). An LTI was a confirmed inhibitor ≥0.60 and <5.00 BU/mL, and an HTI was a confirmed inhibitor ≥5.00 BU/mL. A transient inhibitor was defined as an inhibitor that disappeared spontaneously within 6 months and did not reappear when the patient was reexposed to FVIII coagulation factor product (ie, rFVIIIFc), in accordance with the definition used by the International Society on Thrombosis and Haemostasis23 and in a previous study.24
Any patient who had discrepant inhibitor test results (eg, an initial LTI result followed by an HTI result) was required to have repeat inhibitor testing from a separate sample drawn 2 to 4 weeks after the previous sample, with inhibitor classifications made based on the collective results (ie, if 2 of 3 test results were high or low readings, the patient would be classified as such). Patients on both on-demand and prophylaxis regimens had visits at least every 12 (±2) weeks in which blood samples were taken for inhibitor testing. For patients receiving on-demand treatment who had no infusions of factor since the previous visit, blood draws were only required every 24 weeks rather than every 12 weeks. Inhibitor testing also took place at specific ED milestones (ie, 5 EDs, 10-15 EDs, 20-25 EDs, 50-55 EDs, 75-80 EDs, 100-105 EDs). The study was performed in accordance with the Declaration of Helsinki and all local regulations. Written informed consent was obtained from all patients’ parents/legal guardians.
Post hoc analyses
Post hoc analyses included timing of inhibitor development for all patients who were inhibitor-positive and for HTI and LTI subgroups and timing of inhibitor development by treatment regimen (on-demand vs prophylaxis). We also provided descriptive and statistical analyses of baseline patient demographics and characteristics (age, race, ethnicity, and genotype) and nongenetic risk factors (ie, IFE and CVAD placement and dosing) and descriptive statistics of the LTI cohort regarding inhibitor outcomes (ie, transient, persistent, or progressed to HTI).
Statistical analysis and definitions
Patient demographics were stratified in 2 ways: by inhibitor status (negative vs positive) or by titer level (negative vs high titer or negative vs low titer). Differences in proportions by stratification variable comparing patient demographics (age, race, and ethnicity) as well as genotype risk classification were tested using Fisher exact test. Genotype was classified according to Gouw et al,4 as high-risk variants (inversions, large structural changes [>50 base pairs], frameshift, and nonsense mutations), and low-risk variants (splice site and missense mutations).
Kaplan-Meier survival analyses were used to evaluate time from regimen start to first inhibitor development in months and in EDs by on-demand or prophylaxis treatment regimen.
IFE was defined as ≥1 infusion per day on ≥3 consecutive days. The number (%) of patients with CVAD placement surgery and IFE were investigated in relation to EDs (at first exposure, during first 10 EDs, or during first 20 EDs), as shown in supplemental Table 3. Statistical testing was by inhibitor status using either a 2-sample t test (for mean comparisons) or Fisher exact test (for comparison of proportions). All data regarding IFE in inhibitor patients relate to preinhibitor development. For analyses of factor consumption data, statistical testing was by 2-sided Mann-Whitney U test.
As the analyses presented are descriptive and post hoc, no reference to “statistical significance” was made; however, unadjusted P values have been shown for descriptive and informational reasons without being inferential.
Results
Study population: inhibitor disposition and treatment regimen
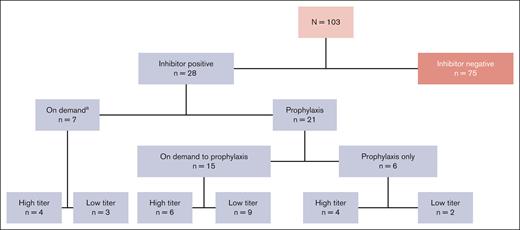
Of the 103 patients who received ≥1 dose of rFVIIIFc, 28 patients developed inhibitors (Figure 1).22 At the time of inhibitor development, 7 of 28 patients (25%) were receiving on-demand therapy and 21 (75%) were receiving prophylaxis treatment. Fifty percent (14/28) of the patients who were inhibitor-positive developed an LTI and 50% developed an HTI.
PUPs A-LONG patient disposition by rFVIIIFc treatment regimen at the time of inhibitor development.aOf those who developed inhibitors while on demand, 2 patients with HTIs and 1 with LTIs subsequently switched regimen from on-demand to prophylaxis.
PUPs A-LONG patient disposition by rFVIIIFc treatment regimen at the time of inhibitor development.aOf those who developed inhibitors while on demand, 2 patients with HTIs and 1 with LTIs subsequently switched regimen from on-demand to prophylaxis.
Of the 14 patients with HTIs, 7 had HTIs at first detection; the remaining 7 initially developed an LTI that subsequently progressed to an HTI. Of the 14 patients with LTIs who never developed HTIs, 7 had persistent LTIs, whereas, an equal number (n = 7) had transient LTIs that resolved spontaneously. Of those on prophylaxis treatment at the time of inhibitor development, 15 of 21 patients (71%) had switched from on-demand therapy to prophylaxis treatment.
Time to inhibitor development and treatment regimen
The individual treatment regimen patterns and time to first positive inhibitor development from first dose on study for the PUPs A-LONG inhibitor population (n = 28) are shown in supplemental Figure 1.
Of patients with HTIs (n = 14), 7 with HTIs at first detection had a median (range) of 9.0 EDs (5.0-12.0) and 8.3 weeks (4.7-67.1) to inhibitor development (supplemental Table 1).
Patients who initially developed an LTI that subsequently progressed to an HTI (n = 7) had a median (range) of 9.0 EDs (4.0-14.0) (8.4 weeks [4.1-46.1]) from first dose on study to LTI development and a median (range) of 8.0 EDs (3.0-16.0) (5.9 weeks [2.1-8.1]) from LTI to HTI development.
Among the 7 patients with persistent LTIs, the median (range) number of EDs to inhibitor development was 11.0 (1.0-53.0) (supplemental Table 1); median (range) time to inhibitor development in weeks was 30.0 (4.3-58.1).
Among the 7 patients who had transient LTIs, the median (range) number of EDs to inhibitor development was 12.0 (8.0-37.0) (16.6 [7.9-38.7] weeks), and the median (range) number of EDs from LTI development to resolution was 31.0 (5.0-38.0) (12.3 [6.1-17.4] weeks).
Inhibitor risk: patient demographics and genotype classification
The median age (range) at the time of enrollment was 7.4 months (0.24-48.0) among patients who remained inhibitor-negative and 6.7 months (0.96-24.0) among those who became inhibitor-positive (Table 1; supplemental Table 2). The distribution of baseline characteristics (age, race, and ethnicity) was similar between inhibitor-positive and inhibitor-negative subgroups in this cohort (all P > .05) (Table 1); however, the relationship between inhibitor subgroups for Black race could not be evaluated due to the small number of patients (n = 2).
Of 78 patients with a high-risk genotype in the overall PUPs A-LONG study population, 10 (12.8%) developed inhibitors during their first treatment regimen (on-demand, n = 4; prophylaxis, n = 6). A greater proportion of patients with HTIs were found to have high-risk variants as compared with those who remained inhibitor-negative (P = .028) (Table 1). Further analysis indicated there was a greater proportion of patients among the HTI group who had large structural change variants (deletions or insertions) or nonsense variants; 5 of 14 patients with HTIs (36%) had a large structural change (>50 base pairs) or nonsense variant, compared with 8 of 75 patients who remained inhibitor-negative (11%) (P = .031).
In patients who developed HTIs, the proportion of patients with a family history of inhibitors was significantly higher (6/12; 50.0%) as compared with patients who did not develop inhibitors (11/67; 16.4%; P = .0176); the proportion with a family history of inhibitors was 25.0% (3/12) in those who developed LTIs.
Nongenetic risk factors
Most patients on prophylaxis (n = 24) developed inhibitors within 2 months after first exposure, whereas those treated on demand (n = 4) largely developed inhibitors within 12 months (supplemental Figure 2A). There were similar rates of inhibitor development in each regimen (prophylaxis vs on-demand) when accounting for EDs (supplemental Figure 2B). For patients who developed inhibitors after a switch from on-demand to prophylactic treatment (n = 15), the mean (standard deviation) total number of EDs accumulated was 3.2 (2.3) for on-demand treatment and 12.2 (13.1) for prophylaxis.
Inhibitor subgroup risk: CVAD placement surgery and IFE
We analyzed the contribution, if any, of CVAD placement surgery and IFE to inhibitor development. The data presented below refer to CVAD insertion and IFE before inhibitor development. It should be noted that after inhibitor development, most young children would have required CVAD insertion to facilitate management of bleeds. Twenty-four patients had a CVAD inserted within the first 10 EDs (excluding any CVAD inserted postinhibitor development); of these, 7 of 24 (29%) went on to develop inhibitors (supplemental Table 3). The median (range) time to inhibitor development after CVAD placement in the 7 patients who developed inhibitors post-CVAD insertion was 21 days (10-41). All 7 patients received on-demand rFVIIIFc as their initial treatment regimen and switched to prophylaxis regimen; inhibitor development occurred during the prophylaxis regimen. Among the 79 patients who did not have a CVAD inserted within the first 10 EDs, 21 (27%) went on to develop inhibitors.
Of 32 patients who received IFE during the first 10 EDs, 9 (28%) developed inhibitors (post-IFE). The mean (standard deviation) daily dose of IFE during the first 10 EDs was 58.1 (21.2) IU/kg for the 9 patients who developed inhibitors and 53.4 (20.3) IU/kg among the 23 patients who did not develop inhibitors (supplemental Table 3). Among the 14 patients with HTIs, only 2 (14%) had IFE preinhibitor development compared with 7 (50%) patients with LTIs (Table 2). For patients who never developed an inhibitor but received IFE at some point during the study (n = 35), the median dose (51.1 IU/kg per day) was significantly lower than those who developed HTIs (65.6 IU/kg per day; n = 2; P = .0002) but not LTIs (53.6 IU/kg per day; n = 7; P = .2656). The median (range) time to inhibitor development after IFE was 26 days (11-117). Five of 9 patients who became inhibitor-positive (2 high titer and 3 low titer) with preinhibitor IFE (56%) received IFE due to CVAD placement (Table 2); the reason for IFE in the other 4 patients included spontaneous joint bleeds and trauma-related muscle, skin/mucosa and internal bleeds (intracranial hemorrhage). In comparison, for the 23 patients who received IFE in the first 10 EDs and did not develop inhibitors (supplemental Table 3), the most common reason for the IFE was preoperative prophylaxis.
Inhibitor titer Bethesda unit analyses
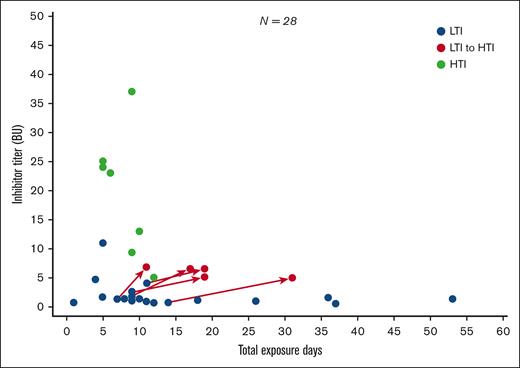
Figure 2 shows inhibitor titer (BU/mL) by EDs in patients with HTIs vs those with LTIs. In total, 21 of the 28 patients who developed inhibitors initially developed LTIs. Of these, 7 resolved spontaneously (ie, were transient), 7 persisted, and 7 progressed to HTIs (supplemental Figure 3). All 7 patients with persistent LTIs and 6 of 7 patients with transient LTIs had a first low-titer value of <2 BU/mL. In contrast, of those who progressed from LTIs to HTIs, 4 of 7 had a first LTI value of <2 BU/mL.
First inhibitor titer (BU/mL) value by EDsa for first LTI and HTI developmentb-e.aBefore inhibitor development or inhibitor progression. bImmune tolerance induction regimen is excluded from this analysis. cTwo patients have no data post LTI/HTI development. dCategorization of titer level requires a positive inhibitor test result followed by a second positive test result 2 to 4 weeks after the original sample was drawn. This analysis is based on the first of the 2 inhibitor readings. The categorization of the patient with LTIs with a value of ∼11 BU at 5 EDs was based on failing to achieve a second repeat inhibitor test above 5 BU/mL needed to confirm HTI classification. eSeven of the patients with HTIs (50%) had previously developed an LTI.
First inhibitor titer (BU/mL) value by EDsa for first LTI and HTI developmentb-e.aBefore inhibitor development or inhibitor progression. bImmune tolerance induction regimen is excluded from this analysis. cTwo patients have no data post LTI/HTI development. dCategorization of titer level requires a positive inhibitor test result followed by a second positive test result 2 to 4 weeks after the original sample was drawn. This analysis is based on the first of the 2 inhibitor readings. The categorization of the patient with LTIs with a value of ∼11 BU at 5 EDs was based on failing to achieve a second repeat inhibitor test above 5 BU/mL needed to confirm HTI classification. eSeven of the patients with HTIs (50%) had previously developed an LTI.
Consumption data for inhibitor subgroups
Matching patients who were inhibitor-positive and inhibitor-negative within 5 EDs indicated no clear difference between the groups in cumulative dose (IU/kg) administered up until inhibitor development (data not shown). Furthermore, no clear differences in preinhibitor exposure were observed between the 14 patients with HTIs and the 14 with LTIs (Table 3).
Patients with LTIs that resolved spontaneously or persisted received a lower median dose per infusion after inhibitor development compared with those whose LTI progressed to HTI; infusions were also more frequent among the transient or persistent LTI subgroups (Table 4). The mean dose per infusion from LTI development until resolution for those with transient LTIs (n = 7) was 59.2 (27.3-94.5) IU/kg, whereas the mean dose per infusion from LTI development until HTI development for patients who progressed to HTIs (n = 5) was 98.4 (81.9-104.4) IU/kg (P = .033). The mean dosing interval was 2.6 (1.9-7.0) days in the transient LTI population (n = 7) and 4.7 (1.3-6.9) days in those who subsequently developed HTIs (all P > .05).
Discussion
Determining risk factors contributing to inhibitor formation is an important area of study because the effective management and treatment of patients with inhibitors remains a high unmet clinical need. These post hoc analyses provide a descriptive view of the PUPs A-LONG inhibitor population and investigated factors that may influence risk of inhibitor development.
Previous studies have demonstrated that individuals with certain F8 variants (intron 22 inversions, large structural changes, frameshift, and nonsense) are more likely to develop inhibitors.4,14,15 In our analyses, the patients who became inhibitor-positive or remained inhibitor-negative showed a similar distribution across the baseline characteristics of age, race, and ethnicity in this cohort. However, we found an association of high-risk variants with HTI development when compared with the inhibitor-negative group. Specifically, we found a correlation of large structural changes and nonsense variants, collectively, with HTI development. Gouw et al observed that, among high-risk variants, the inhibitor risk was higher for large deletions and nonsense mutations compared with intron 22 inversions.14 This study supports the above finding; moreover, it shows an association of these variants with the development of HTIs, suggesting that large structural changes and nonsense variants may contribute mainly to the development of HTIs. Family history of inhibitor development was also found to be a contributing factor to whether a patient developed an inhibitor.
Among the inhibitor group in this cohort, we found that HTI development was often preceded by the presence of an LTI. This occurred in 7 of the 14 patients (50%) who developed HTIs. This is in concordance with a prior study of inhibitor development in children with severe hemophilia A.12 LTIs can progress to high titer after reexposure to FVIII concentrate; thus, inhibitor classification may vary with time.12 Indeed, the increased frequency of, and adherence to inhibitor testing in a clinical trial setting may help identify patients that progress from an LTI to an HTI. Inhibitor testing in the PUPs A-LONG study occurred at specific ED milestones and due to the timing of blood draws, it is possible that initial LTIs may have been missed in patients who had an HTI in the first positive inhibitor sample. This heterogeneity in inhibitor development (some develop, some do not; some develop HTI, some LTI, and some LTI progress to HTI) highlights a limitation in the current understanding of the immunological mechanisms of inhibitor development.
There were no clear differences in nongenetic risk factors between those developing and not developing inhibitors. However, we found that patients with LTIs that progressed to HTIs received a higher dose per infusion than patients in whom LTIs spontaneously resolved or persisted. Patients with transient or persistent LTIs also received more frequent infusions than those whose LTI progressed to HTI. This suggests that frequent low-dose exposure may reduce the risk of progression from LTI to HTI; whereas, infrequent large doses may induce this progression.
High-dose intensive FVIII treatment has been associated with an increased risk of inhibitor formation in PUPs with hemophilia A.17,18,25-28 Our results corroborate existing findings and suggest that prescribing a higher dose of rFVIIIFc in patients with LTIs may increase the risk of HTI development. Mancuso et al12 found that progression to HTIs was associated with F8 genotype and a family history of inhibitors. Concordant with our results, Halimeh et al recommended that pediatric patients at risk of inhibitor formation should be prescribed carefully calculated lower-dose regimens that are tailored to the individual.18
We showed that 7 patients in the PUPs A-LONG inhibitor population had LTIs that resolved spontaneously. This analysis was based on the inhibitor classification used by Tagariello et al,24 who defined inhibitors as “transient” and “persistent” based on the time to inhibitor resolution. Transient inhibitors were defined as those that resolved spontaneously within 6 months and did not reappear on FVIII exposure, and an inhibitor was classified as persistent if the inhibitor remained positive or anamnesis occurred.
In the 2016 Survey of Inhibitors in Plasma-Product Exposed Toddlers, 10 of 17 (59%) patients with LTIs and 12 of 44 (27%) patients had transient inhibitors6; whereas, the study from Tagariello et al found that 64 of 168 (38%) patients who were inhibitor-positive with severe hemophilia A, had transient inhibitors.24 This study reports a similar incidence of transient inhibitors (25% of patients who were inhibitor-positive), although with a smaller study population.
Older reports suggest ∼10% of LTIs are transient. However, the definition of what constitutes a transient inhibitor varies; most studies use <6 months to define transient inhibitors, but resolution after >6 months of inhibitor-positive results may still be considered transient.29 The authors of the Real-life Management of Inhibitors study reported 10 patients who experienced resolution of LTIs without immune tolerance induction (after a median of 6.5 months) and suggest that patients with LTIs could benefit from continuing on standard prophylaxis to achieve negative titers and fully effective replacement therapy.30 Our results support this statement, suggesting that those with lower dosing tended to experience resolution.
Fewer patients with HTIs (n = 2) had IFE compared with the LTI group (n = 7), suggesting that IFE might increase the risk of LTIs but not HTIs. Our analyses suggest that patients with HTIs tended to receive a higher median dose of IFE per day than those with LTI. Previous studies have shown that intensive FVIII treatment is associated with inhibitor development; however, IFE is defined and assessed in different ways, making direct comparison difficult.17,25,31,32
We did not find an association for CVAD placement surgery as a risk factor for inhibitor development. In our cohort, 7 patients went on to develop an inhibitor post-CVAD insertion, whereas 24 patients had a CVAD inserted at some point during the study but never developed an inhibitor. There is evidence that CVAD use and complications resulting from CVAD insertion (eg, infection) are risk factors for inhibitor development2; our analyses did not support this, although further study is required in a larger cohort.
People with hemophilia frequently undergo CVAD placement surgery to facilitate venous access.33 HTI development can often necessitate CVAD placement surgery, particularly in young children. During surgical procedures, intensive factor exposure is often required to maintain hemostasis,34 and intensive treatment initiated as a result of a surgical procedure has been investigated as a potential risk factor for inhibitor development in PUPs with hemophilia A.35,36 However, several studies have found no association between intense treatment associated with CVAD placement surgery and inhibitor development.17,31,37 Although we did not find an association in our study, we report that 5 patients who went on to develop inhibitors received IFE due to CVAD placement (all within 10 EDs), warranting further investigation with a larger sample size. Similar rates of inhibitor development were observed between prophylaxis and on-demand regimens while accounting for EDs. This is consistent with previous studies demonstrating that inhibitor development typically occurs within the first 50 EDs.10 Thus this study did not find that early start of prophylaxis led to a reduction in inhibitor development.
It has been suggested that using recombinant products from nonhuman mammalian cell lines introduces posttranslational protein modifications that are not native in humans, and therefore, immunogenicity may be reduced for proteins produced in human cell lines.38 However, not only have products produced in nonhuman cell lines been successfully used for many years, we observe that the incidence of inhibitors in PUPs treated with rFVIIIFc is similar to the rate of inhibitors in PUPs treated with turoctocog alfa pegol (29.9% overall incidence of inhibitors), the latter of which is produced in Chinese hamster ovary cells.39,40
Limitations of the study include the small patient numbers, although differences were found for genotype and LTI consumption data. Moreover, because the patient population was predominantly White, these results may not be generalizable to other ethnic groups. In addition, genotype risk classification is not clearly defined in the literature and the genotype analyses are based on previous studies rather than an existing consensus in the field. In this study there were 17 patients whose genotype was unknown. If the genotype of these patients had been known, the statistical analysis of genotype as a risk factor for inhibitor development may have been affected.
In conclusion, HTI development tended to occur more frequently in those patients with high-risk F8 variants (particularly large structural changes, such as deletions/insertions or nonsense mutations); whereas, LTI development was more prevalent after IFE. Finally, the natural progression of LTIs may be influenced by the subsequent treatment regimen, notably the dose but also the frequency, and this warrants further investigation. In summary, these findings may help guide dosing for patients with hemophilia A with inhibitors and suggest it may be beneficial to stratify treatment strategies according to inhibitor risk.41 Further identifying factors that predispose individuals to inhibitor development remains a priority.
Acknowledgments
The investigators and Sanofi and Sobi gratefully thank the patients and their families and all investigators and their teams for participating in the PUPs A-LONG study.
This study was funded by Sanofi (Cambridge, MA) and Sobi (Stockholm, Sweden). Medical writing support and editorial assistance for the development of this manuscript was provided by Carissa Drake and Ashleigh Pulkoski-Gross, Fishawack Communications Ltd, part of Avalere Health, and was funded by Sanofi and Sobi.
Authorship
Contribution: M.C. contributed substantially to the design of the work; M.C. and C.K. contributed substantially to data acquisition; M.C., M.S., E.S., C.K., R.K., P.R., M.F., and S.C. contributed substantially to data analysis and data interpretation; and all authors had full editorial control and provided critical revision of the manuscript and approved the content for publication.
Conflict-of-interest disclosure: M.C. has received research support and/or honoraria for speaking/participating in advisory boards from Bayer, Bioverativ (a Sanofi company), Laboratoire français du Fractionnement et des Biotechnologies (LFB), Novo Nordisk, Novartis, Pfizer, Roche, and Takeda. M.S. has received personal/speaker fees from Bayer, CSL Behring, Kedrion, Roche/Chugai, and Sobi. M.S. has also served on advisory boards for CSL Behring, Novo Nordisk, Roche/Chugai, and Sobi. R.K. has served on advisory boards for Sanofi, Novo Nordisk, Pfizer, and CSL Behring. R.K. has also received clinical trial research grants from Sanofi, BioMarin, ApcinteX/Centessa, and Novo Nordisk. P.R., M.F., and S.C. are employees of Sanofi and may hold shares and/or stock options in the company. E.S. is an employee of Sobi and may hold shares and/or stock options in the company. C.K. has received personal fees from BFSH, CSL Behring, MSD, Novo Nordisk, Roche/Chugai, Sobi/Sanofi, and Takeda. C.K.’s institution has also received grants from Bayer Vital GmbH, Biotest, CSL Behring, Intersero, Novo Nordisk, Pfizer, Roche/Chugai, Sanofi/Sobi, Takeda, the European Union (H2020), and federal funding.
Correspondence: Manuel Carcao, Hospital for Sick Children, University of Toronto, 555 University Avenue, Toronto, ON M5G 1X8, Canada; email: manuel.carcao@sickkids.ca.
References
Author notes
Qualified researchers may request access to data and related documents. Any patient-level data will be anonymized, and study documents will be redacted, including to protect the privacy of trial patients. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://vivli.org/.
The full-text version of this article contains a data supplement.