Using the PRO-RBDD, comprehensive clinical and genotype features of 123 patients with CFD were evaluated.
Bleeding severity grades agreed with the factor activity threshold in nearly half of cases with quantitative defects.
Visual Abstract
Congenital fibrinogen deficiency (CFD) is a rare bleeding disorder caused by mutations in FGA, FGB, and FGG. We sought to comprehensively characterize patients with CFD using PRO-RBDD (Prospective Rare Bleeding Disorders Database). Clinical phenotypes, laboratory, and genetic features were investigated using retrospective data from the PRO-RBDD. Patients were classified from asymptomatic to grade 3 based on their bleeding severity. In addition, FGA, FGB, and FGG were sequenced to find causative variants. A total of 166 CFD cases from 16 countries were included, of whom 123 (30 afibrinogenemia, 33 hypofibrinogenemia, 55 dysfibrinogenemia, and 5 hypodysfibrinogenemia) were well characterized. Considering the previously established factor activity and antigen level thresholds, bleeding severity was correctly identified in 58% of the cases. The rates of thrombotic events among afibrinogenemic and hypofibrinogenemic patients were relatively similar (11% and 10%, respectively) and surprisingly higher than in dysfibrinogenemic cases. The rate of spontaneous abortions among 68 pregnancies was 31%, including 86% in dysfibrinogenemic women and 14% with hypofibrinogenemia. Eighty-six patients received treatment (69 on-demand and/or 17 on prophylaxis), with fibrinogen concentrates being the most frequently used product. Genetic analysis was available for 91 cases and 41 distinct variants were identified. Hotspot variants (FGG, p.Arg301Cys/His and FGA, p.Arg35Cys/His) were present in 51% of dysfibrinogenemia. Obstetric complications were commonly observed in dysfibrinogenemia. This large multicenter study provided a comprehensive insight into the clinical, laboratory, and genetic history of patients with CFDs. We conclude that bleeding severity grades were in agreement with the established factor activity threshold in nearly half of the cases with quantitative defects.
Introduction
Coagulation proteins are the core components of the coagulation cascade, which leads to a complex interplay of reactions that prevent blood loss by converting soluble fibrinogen to insoluble fibrin strands through the interaction with platelets. Fibrinogen is a 340 KDa glycoprotein that is produced in the liver and circulates in blood at 150 to 450 mg/dL plasma concentrations.1 This glycoprotein consists of 2 sets of 3 polypeptide chains, called alpha (α), beta (β), and gamma (γ), which are encoded by the FGA, FGB, and FGG genes on chromosome 4.2 Congenital fibrinogen disorders (CFD), caused by mutations in these genes leading to fibrinogen deficiency and/or dysfunction,3 are classified as quantitative (type 1) or qualitative (type 2) deficiencies based on fibrinogen plasma levels. Quantitative deficiencies are characterized by the complete lack of fibrinogen in plasma (afibrinogenemia) or by a proportional decrease (hypofibrinogenemia) of fibrinogen coagulant activity (Fg:C) and antigen levels (Fg:Ag).4 Qualitative deficiencies are described by normal Fg:Ag and decreased Fg:C (dysfibrinogenemia) or by a disproportional decrease of both Fg:C and Fg:Ag levels (hypodysfibrinogenemia).4 Afibrinogenemia, the rarest fibrinogen deficiency, has an estimated prevalence of 1 in 1 million but is more frequent in countries where consanguineous marriages are common and is often diagnosed in the newborn period.5,6 Patients with congenital afibrinogenemia show severe bleeding symptoms, mostly manifesting in the neonatal period with umbilical cord bleeding or after circumcision.7 Afibrinogenemic cases predominately experience bleeding but may also experience thrombotic events with or without replacement therapy, mainly because of the impairment of the antithrombin function of fibrin and related inability to sequester thrombin.8-10 Inherited hypofibrinogenemia is associated with a mild bleeding tendency and more rarely by thrombotic complications.11 Most patients with inherited dysfibrinogenemia are asymptomatic and are usually identified through abnormal blood coagulation tests or their family history. However, some patients may experience bleeding (rarely spontaneously) or thromboembolic complications due to preventing thrombin from binding to fibrin, thus leading to an increased level of free thrombin in the blood.12,13 Additionally, thrombosis can also occur when the fibrin clot strength, structure, or stability are altered.14 Because fibrinogen plays a major role during pregnancy, women with CFD are at increased risk for adverse obstetric outcomes11 related to the fact that fibrinogen plays an essential role in maintaining the placenta, supporting early-term trophoblast proliferation and spreading, and supporting the maternal-fetal circulation.15-17
The severity of clinical manifestations is dependent on the fibrinogen activity plasma level. In a retrospective study from the European Network of Rare Bleeding Disorders (EN-RBD), including 46 patients with fibrinogen deficiency, patients with a mean fibrinogen activity level of at least 70 mg/dL appeared to be protected from spontaneous bleeds, whereas a level >100 mg/dL completely protected the individual from any bleeding symptoms.18 Because the clinical assessment of hemorrhagic symptoms is an important step in the evaluation of patients, International Society on Thrombosis and Hemostasis Bleeding Assessment Tool (ISTH-BAT) and the EN-RBD-Bleeding Score System (EN-RBD-BSS) have been established to standardize the assessment of bleeding symptoms; these can help distinguish individuals affected by a bleeding tendency from those unaffected.19-22 According to the study by the EN-RBD, patients with rare bleeding disorders can be classified as asymptomatic, grade 1, grade 2, or grade 3 based on locations, potential clinical impact, and spontaneity of bleeding.18 In the same study, the established threshold of fibrinogen activity level necessary to prevent bleeding was above 100 mg/dL and an undetectable fibrinogen level corresponds to the presence of major spontaneous bleeding (grade 3).18
Genetic variants of FGA, FGB, and FGG are useful for the correct diagnosis and to identify its types and subtypes as well.23 More than 250 causative mutations have been reported in online databases associated with CFD.24,25 Heterozygous or homozygous mutations in the 3 fibrinogen genes can lead to congenital quantitative fibrinogen disorders.26 Almost all cases of afibrinogenemia are due to null mutations (ie, nonsense mutations, splice site mutations, frameshift mutations, and large deletions) and are mostly located in FGA. In hypofibrinogenemia, heterozygous missense mutations are more frequent and distributed throughout the 3 genes. In most dysfibrinogenemic cases, the disease is caused by heterozygous missense mutations in FGA and FGG; hypodysfibrinogenemic patients are heterozygous or combined heterozygous, and their causative mutations are mainly located in FGG.27 Despite the relative rarity of CFD, the number of cases reported so far is substantial.14,25,27 Nonetheless, combining the results obtained from laboratory phenotype, genotype, and clinical analyses is warranted to yield valuable information on the development and course of these diseases as well as for the choice of the appropriate treatment. Therefore, the establishment of international collaborations and registries are key steps that help to improve patient management.
Considering this background, we performed a cross-sectional study within the Prospective Rare Bleeding Disorders Database (PRO-RBDD) with the goal of describing the laboratory, clinical and genetic features of a well-defined group of patients with CFD. In addition, we aimed to assess the association between factor activity level and clinical severity in a more comprehensive way to confirm the EN-RBD results in a wider and different cohort of patients.18 Another aim was to find the difference between the age of diagnosis among different types of fibrinogen deficiency. This study was designed within the European Haemophilia Network activities with the support of the European Association for Haemophilia and Allied Disorders and the European Commission Health Programme through the Executive Agency for Health and Consumers.28
Methods
Data collection
This analysis was performed using data retrieved at baseline from the PRO-RBDD (demographic data and clinical historical data from the date of birth to the enrollment date), with a cross-sectional design.29 A total of 166 records of patients with CFD were collected and entered into the database from February 2013 to 2020. Data were available on demographics (age at data collection and sex), fibrinogen coagulant activity, and antigen levels (not at the time of treatment), lifelong clinical histories up to the date of inclusion into the study, age at first bleeding, age at diagnosis, reason for diagnosis, and types of treatment. Cases were evaluated in relation to clinical severity defined as asymptomatic (ie, no documented bleeding episodes), grade 1 (ie, bleeding after trauma or drug-induced), grade 2 (ie, spontaneous minor bleeding), and grade 3 (ie, spontaneous major bleeding).18 Data were collected from 36 centers in 16 countries, including Italy (n = 38), the United Kingdom (n = 14), The Netherlands (n = 12), Pakistan (n = 11), Greece (n = 8), Turkey (n = 7), the United States (n = 6), Iran (n = 5), Germany (n = 5), France (n = 4), Switzerland (n = 4), Czech Republic (n = 4), Colombia (n = 2), Bosnia and Herzegovina (n = 1), Serbia (n = 1), and Indonesia (n = 1). The project was approved by the ethical review board of the Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy and written informed consent was obtained from all enrolled patients or their parents.
Laboratory assays
The historical diagnosis of fibrinogen deficiency was based on coagulant activity levels below the normal threshold of each laboratory; however, plasma samples and blood cells from most cases were shipped for diagnosis confirmation and genetic analysis to central laboratories (Laboratory of Molecular Pathology of the Angelo Bianchi Bonomi Haemophilia and Thrombosis Centre, Milan, Italy, and Division of Angiology and Haemostasis University Hospitals of Geneva, Geneva, Switzerland). Fg:C was measured by the Clauss method and Fg:Ag level by the turbidimetric latex immunoassay (HYPHEN BioMed, LIAPHEN, Neuville-sur-Oise, France). Most patients were classified based on the activity/antigen ratio that was used to distinguish between hypofibrinogenemia and dysfibrinogenemia (the ratio is Fg:C/Fg:Ag <0.7 in dysfibrinogenemia and is Fg:C/Fg:Ag >0.7 in hypofibrinogenemia).30 Half of the lowest detection limit was considered to represent a complete deficiency to facilitate analysis relating to factor activity levels (ie, 5 mg/dL for a detection limit of 10 mg/dL in afibrinogenemic cases).
Genetic analysis
Genomic DNA was amplified by polymerase chain reaction under standard conditions using sense and antisense primers designed on the basis of known sequences of the fibrinogen cluster. Direct sequencing of amplified fragments was performed by Sanger sequencing. The Human Gene Mutation Database (https://www.hgmd.cf.ac.uk/ac/index.php) and the Leiden Open Variation Database (https://www.lovd.nl/3.0/home) were used to find the already reported variants associated with CFD. Novel variants were evaluated using in silico prediction by Polymorphism Phenotyping (PolyPhen; http://genetics.bwh.harvard.edu/pph2/), Sorting Intolerant From Tolerant (SIFT; http://provean.jcvi.org/), Combined Annotation Dependent Depletion (CADD; https://cadd.gs.washington.edu/) and VarSome (https://varsome.com/) software classification and varSEAK (https://varseak.bio/) was used to predict splice site variants.
Statistical analysis
The Kruskal-Wallis test was used to compare (A) median ages at diagnosis between the type of fibrinogen deficiencies and (B) median factor activity levels between the grade of bleeding severity, P value <.05 being considered statistically significant. Pairwise comparisons between group levels with corrections for multiple testing were calculated (correction by Benjamini, Hochberg). Sensitivity, specificity, and positive predictive value (precision) were evaluated for established factor activity thresholds in quantitative fibrinogen deficiency. Statistical analysis was performed using IBM SPSS Statistics 26 and R Studio version 4.0.3.
Results
Patient data
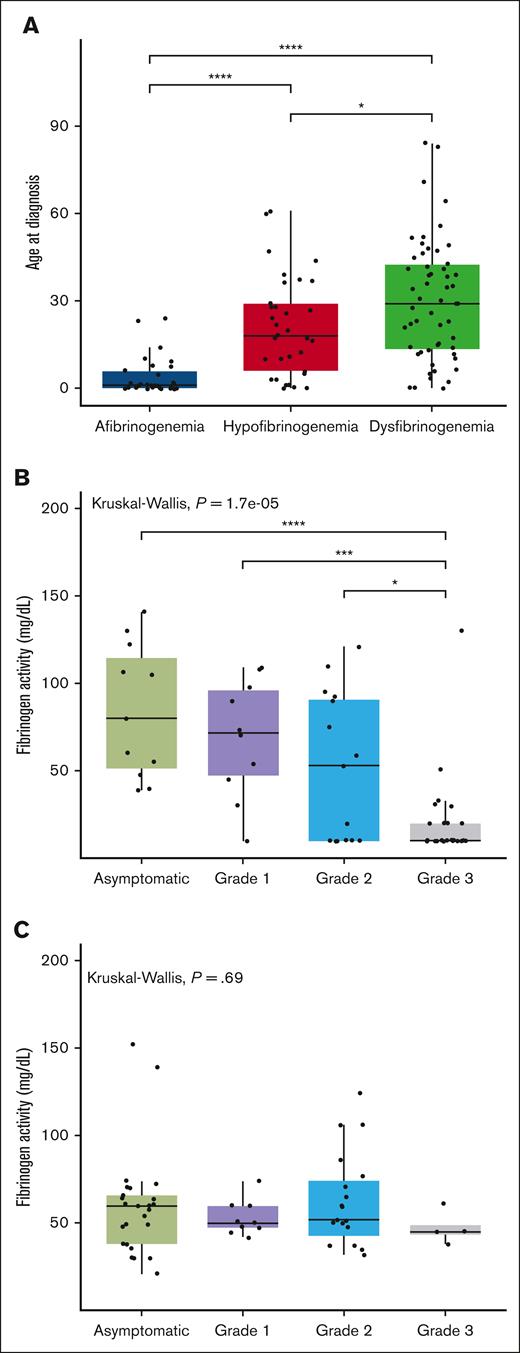
Out of the 166 cases recorded in the PRO-RBDD, 123 (80 adults and 43 children) had available results for both Fg:C and Fg:Ag, so all analyses were performed on this population. The demographic data and laboratory results are shown in Table 1. Patients were classified as afibrinogenemia (n = 30, 24%), hypofibrinogenemia (n = 33, 27%), dysfibrinogenemia (n = 55, 45%), and hypodysfibrinogenemia (n = 5, 4%). The median (range) age of cases at study inclusion was 26 (0-85) years. The median age at diagnosis was significantly lower in afibrinogenemic patients, with a median age of 1 year (0-24), than in other groups whereas the highest was recorded in cases with dysfibrinogenemia (median, 29 [0-84]). After excluding cases with hypodysfibrinogenemia (because of insufficient data available for separate analyses, n = 5), we confirm a statistically significant difference among the median ages at diagnosis of the 3 CFD subgroups (Figure 1A).
Comparison between age at diagnosis in different groups of fibrinogen deficiencies and the association between factor activity levels and grades of severity. (A) The box plot compares the median age at diagnosis in 3 different fibrinogen deficiency groups. The solid line indicates the median. (B-C) The box plot compares the median factor activity levels between all grades of severity in quantitative (B) and qualitative (C) deficiencies. The Kruskal-Wallis test was performed to determine differences in the age at diagnosis between different subclasses of fibrinogen disorders. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
Comparison between age at diagnosis in different groups of fibrinogen deficiencies and the association between factor activity levels and grades of severity. (A) The box plot compares the median age at diagnosis in 3 different fibrinogen deficiency groups. The solid line indicates the median. (B-C) The box plot compares the median factor activity levels between all grades of severity in quantitative (B) and qualitative (C) deficiencies. The Kruskal-Wallis test was performed to determine differences in the age at diagnosis between different subclasses of fibrinogen disorders. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
Bleeding severity
More than 70% of afibrinogenemic patients had grade 3 bleeding, whereas the majority of those with dysfibrinogenemia and hypofibrinogenemia were asymptomatic (Table 1). An agreement of the bleeding severity grades with the established factor activity threshold in quantitative fibrinogen deficiencies was observed in 36 of 62 cases, that is, with an accuracy of 58% (95% confidence interval, 44.85-70.49). Taking the severity grades as a reference, 45% of those asymptomatic had factor activity levels above 100 mg/dL and 56% of grade 1-2 cases had activity levels between 20 and 100 mg/dL. In patients with grade 3 bleeding, 65% had factor activity levels below 20 mg/dL (Table 2). Except grade 3 in quantitative fibrinogen deficiencies, there were no significant differences between median levels of factor activity for other bleeding severity grades (Figure 1B).
Accordingly, in quantitative fibrinogen deficiencies, the highest sensitivity was found for grade 3 (65%), followed by grade 1-2 (56%) and asymptomatic (45%), whereas the highest specificity (90%) was found for asymptomatic cases (Table 3). Additionally, we were interested in determining the positive predictive value (precision) of established thresholds for different bleeding grades. Our analysis showed that the highest precision was observed in cases with Fg:C <20 mg/dL (71%), which means that 71% of patients with this value were correctly diagnosed as a grade 3.
We further investigated the association between fibrinogen activity and bleeding severity in quantitative fibrinogen deficiencies (afibrinogenemia and hypofibrinogenemia), that resulted in a significant association (P < .0001; Figure 1B), at variance with qualitative deficiencies (P = .69; Figure 1C).
Reasons for diagnosis
All patients with afibrinogenemia and most of those with hypofibrinogenemia were identified due to bleeding episodes, whereas most of the patients with dysfibrinogenemia were diagnosed upon screening for surgery/other clinical question (42%) or as relatives of an affected case (31%), with only 11% due to bleeding (Table 1).
Clinical manifestations
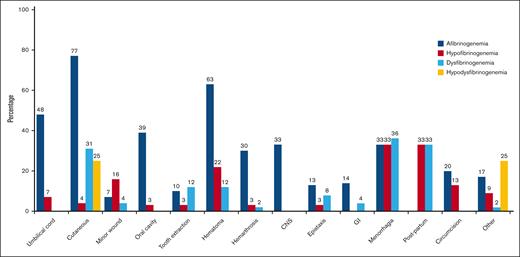
A total of 71% of cases had bleeding episodes at least once in their lives. Figure 2 illustrates the bleeding manifestations in relationship to the different classifications of CFD. Cutaneous bleeding was the most common bleeding symptom (77%) in afibrinogenemic cases followed by hematoma (63%) and umbilical cord bleeding (48%). Of note, hematoma, central nervous system bleeding, and hemarthrosis were significantly more frequent in afibrinogenemic cases (63%, 33%, and 30% respectively). Menorrhagia (36%, 33%) and postpartum hemorrhage (33%, 33%) were the most prevalent manifestations in dysfibrinogenemic and hypofibrinogenemic cases (Figure 2). Nine patients in our study experienced thrombosis (3 with afibrinogenemia, 3 with hypofibrinogenemia, and 3 with dysfibrinogenemia), of which 56% were venous and 44% arterial.
Reported frequency of different types of bleeding symptoms. Menorrhagia was considered in women who were in fertile age, and postpartum bleeding was assessed in women who underwent labor. GI, gastrointestinal bleeding.
Reported frequency of different types of bleeding symptoms. Menorrhagia was considered in women who were in fertile age, and postpartum bleeding was assessed in women who underwent labor. GI, gastrointestinal bleeding.
Pregnancies and complications
Of the 57 (46%) adult women included in this study, 32 (56%) had 68 pregnancies (1 afibrinogenemia, 16 hypofibrinogenemia, 49 dysfibrinogenemia, and 2 hypodysfibrinogenemia) resulting in 47 (69%) live birth (1 afibrinogenemia, 13 hypofibrinogenemia, 31 dysfibrinogenemia, and 2 hypodysfibrinogenemia). A high rate of spontaneous abortions occurred among adult women (21 in 68 pregnancies, 31%) including 18 (86%) in dysfibrinogenemia and 3 (14%) in hypofibrinogenemia. Regarding spontaneous abortions, 2 cases experienced three abortions, 3 cases experienced two and 9 cases experienced one abortion each, with 14 out of 16 (87%) abortions occurring in the first trimester of gestation (there were 5 missing data). Among 14 women who had a spontaneous abortion, 11 (78%) had at least 1 live birth. Postpartum hemorrhage was observed in 10 cases of which 70% were dysfibrinogenemic and 30% were hypofibrinogenemic. Two dysfibrinogenemic cases (10%) had bleeding during pregnancy.
Treatment
A total of 86 patients (56 females and 30 males) received treatment, including 69 on-demand and/or 17 with prophylaxis. Table 1 shows that all afibrinogenemic and hypodysfibrinogenemic cases and more than 60% of those dysfibrinogenemic and hypofibrinogenemic cases received treatment, most of them only on-demand treatment (78%). Prophylaxis was reported in 40% of afibrinogenemic and in 19% of dysfibrinogenemic cases. The majority of cases who received prophylaxis were living in Europe (13, 81%) and in the United States (2, 13%), and most were women (71%) and 59% of them had afibrinogenemia. Fibrinogen concentrate (FC) was the most frequent product used in afibrinogenemic (54%), hypofibrinogenemic (65%), and hypodysfibrinogenemic (100%) cases, whereas dysfibrinogenemic cases mainly received antifibrinolytic drugs (50%). Among afibrinogenemic patients on prophylaxis, 7 patients received FC, 4 cryoprecipitate, and 4 FFP. Furthermore, all dysfibrinogenemic patients on prophylactic treatment were prescribed antifibrinolytic drugs.
Mutational spectrum
As a result of mutational screening using Sanger sequencing of FGA, FGB, and FGG; 41 causative variants were found in 91 patients (Table 4). Among them, 3 variants were novel including 1 in afibrinogenemia (c.115-610G>C, FGB), 1 in hypofibrinogenemia (p.Ser142Ter, FGG), and 1 in hypodysfibrinogenemia (p.Asp346His, FGG). Table 5 summarizes the potential pathogenic effect of the novel variants using the different in silico tools.
In afibrinogenemia and hypofibrinogenemia
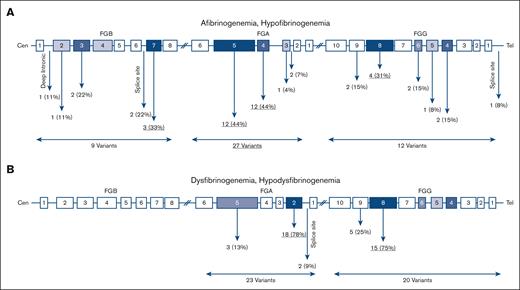
In 23 afibrinogenemic patients 18 different variants were identified of which 13 (72%) were null mutations (ie, nonsense and frameshift), 2 (11%) missense, 2 splice site (11%), and 1 (6%) intronic (Figure 3A). Of note, all cases with afibrinogenemia were homozygous. Mutations were found in FGA, FGB, and FGG in 66%, 22%, and 12% of cases, respectively; and exon 5 FGA was a hot spot that embedded 39% of the mutations.
Distribution of causative variants identified in this study. Location of variants in quantitative (A) and qualitative (B) fibrinogen deficiencies.
Distribution of causative variants identified in this study. Location of variants in quantitative (A) and qualitative (B) fibrinogen deficiencies.
Out of 25 hypofibrinogenemic patients with available genetic results, 20 (80%) cases were heterozygous, 3 (12%) were homozygous, and only 1 case was compound heterozygous for 2 variants. Among 12 different causative variants, missense mutations were the most common (67%), even though 2 (16%) null mutations were also identified. Six (50%) variants were located on FGG.
In dysfibrinogenemia and hypodysfibrinogenemia
All cases with dysfibrinogenemia (n = 39) were heterozygous for a missense mutation in FGA and FGG except for 3 cases in whom a heterozygous frameshift variant was identified in FGA: p.Gly471TrpfsTer3. There were 10 different identified variants in dysfibrinogenemia, all located in FGA (exon 2, 50%) and FGG (exon 8, 30%). The majority of variants (90%) were missense; however, 1 frameshift variant (10%) was also found. Almost 51% of identified variants in dysfibrinogenemia were hot spot mutations located at residues Arg301 in exon 8 of FGG (n = 13, 34%) and at Arg35 in exon 2 of FGA (n = 7, 18%). Of note, the p.Arg38Gly, FGA variant was also frequent, being identified in 6 cases (15%). In hypodysfibrinogenemic cases (n = 4) three different variants were identified, 2 of them in exon 2 and intron 2 of FGA, and 1 in exon 8 of FGG. Two hypodysfibrinogenemic cases carried heterozygous missense variants (p.Cys55Arg, FGA and p.Asp346His, FGG), and the remaining 2 cases heterozygous splice site variants, c.180+1G>C, FGA (Table 4).
Among the 21 cases who experienced spontaneous abortions, 6 had genetic results. Three of them (50%) carried the p.Arg301Cys, FGG mutation and 2 of them had two spontaneous abortions and 1 case had one. A woman with this variant had a live birth. The p.Arg301Cys variant was identified in 2 of 9 patients (22%) who developed thrombosis.
Discussion
This multicentric study is one of the most comprehensive ever conducted to describe the bleeding frequency, treatment products, complications, and genetics stemming from a large international cohort of patients with CFD. According to our results, the bleeding severity of nearly half of the patients was correctly identified on the basis of the established threshold of Fg:C.18 In both previous Iranian and Dutch studies, the established fibrinogen activity level correctly identified the bleeding severity in more than 60% of cases in agreement with our results (58%).31,32 Given that the sensitivity of the bleeding severity grades was low for asymptomatic and grade 1-2 (45% and 56% respectively), further studies with larger cohorts are warranted to establish factor activity level thresholds. Compared with the other rare bleeding disorders, there is a significant association between bleeding severity and Fg:C levels in quantitative fibrinogen deficiency,18 which is indeed confirmed by the present findings (P < .0001). In contrast, no strong association was found in qualitative deficiencies (P = .69; Figure 1C), which could indicate that other underlying variables contributed to the clinical bleeding phenotype in this group.
In our cohort, there was a significant difference between the age at diagnosis among CFD classifications, indicating that afibrinogenemic patients were diagnosed at a younger age (median 1), but those with dysfibrinogenemia were principally diagnosed in adulthood (median 29). The difference between median ages among CFD groups can show that patients with afibrinogenemia often experienced severe bleeding that led to diagnosis at an early age. Early diagnosis and adequate treatment can prevent life-threatening bleeding in afibrinogenemia. In contrast, hypo- or dysfibrinogenemia typically occurs in older adults because of their good prognosis. Regarding bleeding manifestations, the most common symptom in our afibrinogenemic patients was cutaneous bleeding (77%), which was close to the results reported by Sumitha et al (65%).33 However, in contrast to another study, umbilical cord bleeding was not the most common bleeding symptom (85% vs 48%).34 In addition, in our recent study, the incidence rate of umbilical cord bleeding and cutaneous bleeding was almost the same (47%).27 Due to the wide variety of bleeding manifestations in patients with CFD, it is difficult to determine which symptoms are the most frequent.27,35 Nonetheless, women with hypofibrinogenemia and dysfibrinogenemia are more prone to suffer from obstetric complications.36 Of note, these complications are more frequent in afibrinogenemic patients when treatment products are not available. A third of our women experienced spontaneous abortions, which was within the reported range (20%-50%) for hypofibrinogenemia and dysfibrinogenemia.14,36
Thrombosis events occur less frequently in hypofibrinogenemic patients than in afibrinogenemic patients. The reduced amount of circulating fibrinogen in hypofibrinogenemia is thought to reduce the risk of thrombosis, which is more prevalent in afibrinogenemia37,38,39. In the absence of fibrin, the antithrombin action of fibrin is reduced, which could increase circulating thrombin levels and cause thrombosis.39,40 Although thrombosis prevalence has been more frequently reported in qualitative fibrinogen deficiencies,41 the thrombotic events were surprisingly similar among our afibrinogenemic and hypofibrinogenemic cases and higher than in dysfibrinogenemic cases. These findings are comparable with those of a recent study by Hugon-Rodin et al.42 It is unclear whether replacement therapy can lead to thrombotic events, because some studies have reported that thrombosis is associated with replacement therapies in CFD, but others believe that treatment entails a low risk of thrombosis for CFD patients.8,40,43-45 In this study, among 19 patients who received prophylaxis, there was only 1 afibrinogenemic patient (5%) who developed thromboembolism after FC.
Even though 73% of the afibrinogenemic patients were severe, only 40% received prophylaxis. This can be explained by the fact that despite the important efforts to better assess the epidemiology and molecular mechanisms leading to CFD, data on clinical management are still scarce. Indeed, owing to the rarity of these diseases and the absence of randomized controlled studies, recommendations and guidelines are mainly based on expert consensus rather than on evidence-based data. Another explanation for the avoidance of prescribing prophylaxis may be limited access to fibrinogen products in countries with high rates of afibrinogenemia.
A total of 41 different gene variants were identified in this cohort. In afibrinogenemia, the majority of disease-causing variants were nonsense (72%) followed by frameshift (11%) and missense (11%) variants. The identification of c.115-600A>G variant in the FGB intron 1 was a cause of afibrinogenemia as already reported by Davis et al, demonstrating the value of screening deep intronic regions.46 Accordingly, following a screening of this region, we identified a novel c.115-610G>C variant in an afibrinogenemic case with grade 3 bleeding.
The novel p.Asp346His, FGG variant was found in a hypodysfibrinogenemic case with mild bleeding symptoms. Using the UniProt database, this variant was found to impair fibrin polymerization by altering calcium binding.47 In the present study, a 36-year-old hypofibrinogenemic patient with compound heterozygous mutations (c.1417G>A, FGA and c.1244+1G>T, FGB) developed thrombosis but never bled. Nevertheless, c.1244+1G>T, FGB was reported in an afibrinogenemic case with spontaneous bleeding.25
In dysfibrinogenemic cases, hotspot variants (p.Arg301Cys/His, FGG and p.Arg35Cys/His, FGA) were identified in 51% of them in agreement with previous reports.25 Most patients with fibrinogen deficiency who carry the variants in the Arg301 residue do not experience any thrombotic event. It is suggested that the combination of a factor V Leiden defect and the p.Arg301Cys may predispose to thromboembolism.48 In a study in which 52 dysfibrinogenemic cases had variants in Arg301, FGG, 26% and 27% of p.Arg301His and p.Arg301Cys carriers developed thrombosis, indicating no significant association with the risk of thrombosis or major bleeding was reported.14 We found a tendency to both bleeding and thrombosis in 20% of the cases with p.Arg301Cys, FGG, although all the 3 cases with p.Arg301His, FGG had only bleeding. Thus, there is no conclusive evidence that the genotype of these variants contributes to the thrombosis phenotype. We had 6 cases with dysfibrinogenemia carrying p.Arg35His who demonstrated clinical manifestations ranging from asymptomatic to grade 2. In other studies, cases with this variant showed bleeding as well as thrombosis.14,49 For example, Castaman et al reported a patient with menorrhagia who had an ischemic stroke at an older age.49 In contrast, those cases were asymptomatic in another study.25 Altogether, these results indicate the difficulty of treating CFD due to its heterogeneous clinical manifestations. The only case carrying p.Arg35Cys, FGA was asymptomatic in contrast with the previous study by Casini et al.25 Regarding the Arg38, FGA residue, even though the p.Arg38Gly and p.Arg38Ser variants have been reported to be associated with bleeding,50-52 in this study 3 out of 7 (43%) dysfibrinogenemic cases were asymptomatic. For those women who experienced spontaneous abortions, genetic results were available for 6 cases in whom the variants in 2 residues were recurrent (p.Arg301Cys, FGG in 50%, p.Arg38Ser/Gly, FGA in 33%). Similarly, women who suffered postpartum hemorrhage all carried p.Arg301Cys/His, FGG or p.Arg38Gly, FGA. Overall, among 10 women with obstetrical complications (spontaneous abortion, postpartum hemorrhage, and bleeding during pregnancy), 5 cases (50%) were carriers of p.Arg301Cys/His, FGG; 40% were carriers of p.Arg38Ser/Gly, FGA; and only 1 case was carrier of p.Ser384Cys, FGG.
One of the limitations of our study concerns the lack of detailed data regarding thrombosis cases, the outcomes of treatments, and the results of the ISTH-BAT scores. Owing to referral bias, it is reasonable to suspect that not all patients have been entered into the database, so that the present population might be enriched by patients with more severe symptoms. In addition, there were a limited number of hypodysfibrinogenemic cases. Moreover, we have limited data to know whether patients who experienced bleeding episodes were on treatment or were all patients studied before any treatment. It is worth noting that the effects of potential common variants that may change the fibrinogen level and severity cannot be neglected. On the other hand, the strength of this study lies in the international collaborative network which created a web database with a large population to comprehensively collect the clinical phenotype and genotype data of patients with CFD, even though addressing these research questions requires larger cohorts.
In conclusion, this large multicenter study provided a comprehensive insight into the clinical, laboratory, and genetics of patients with CFDs. The findings confirm that the bleeding severity characterizations developed by EN-RBDD18 were concordant with the established factor activity threshold in nearly half of the patients with quantitative defects. The rate of thrombosis following prophylaxis with replacement therapy in the present study was 5%, but further studies are needed. Furthermore, obstetric complications are commonly observed in dysfibrinogenemic patients.
Acknowledgments
The Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico is a member of the European Reference Network EuroBloodNet. The authors would like to thank all participants and physicians who provided cases to be studied. The authors acknowledge Paolo Lanzi and those who constructed the database. In addition, the authors would like to acknowledge P. M. Mannucci for his critical advice in the preparation of this manuscript and L. F. Ghilardini for the illustration work.
This study was part of the European Haemophilia Network (EUHANET) project, supported by the European Commission through its Executive Agency for Health and Consumers. The EUHANET project also received funding from Bayer, Biotest, BPL, CSL Behring, Grifols, Kedrion, LFB, Novo Nordisk, Octapharma, Pfizer, Shire (Baxalta), and Sobi. The Prospective Rare Bleeding Disorders Database project is also funded by the European Association for Haemophilia and Allied Disorders. In addition, this research was partially supported by the Italian Ministry of Health (Bando Ricerca Corrente).
Authorship
Contribution: F.P., R.P., and M.M. designed and constructed the database in collaboration with Paolo Lanzi; S.M., F.P., R.P., and M.M. designed the study; S.M. extracted and analyzed the data and wrote the manuscript; R.P. and M.M. contributed to writing the manuscript; S.M. and S.S. performed statistical analysis; A.L. and A. Casini performed the phenotypic tests; M.N.A., R.A., and A. Cairo performed the genetic analysis; F.P. critically revised the manuscript; all authors have read and approved the final manuscript.
Conflict-of-interest disclosure: F.P. reports participation at educational meetings/symposia of Takeda/Spark and the advisory board of Sanofi, Sobi, CSL Behring, Roche, and BioMarin. R.P. reports participation and speaker fees at the educational workshop organized by Novo Nordisk. S.H. has received speaker’s honorarium from Bayer Healthcare GmbH, Baxalta Innovations (Now Shire) GmbH, Biotest AG, CSL Behring GmbH and Novartis Pharma GmbH, Novo Nordisk Pharma, GmbH, Octapharma GmbH, Pfizer Pharma, Roche Pharma AG, Swedish Orphan Biovitrum GmbH; and received research grant and attended advisory board of Bayer Healthcare GmbH, Biotest AG, CSL Behring GmbH, Novo Nordisk Pharma, GmbH, Octapharma GmbH, Chugai Pharma Germany GmbH, and Swedish Orphan Biovitrum GmbH. J.B. has received speaker’s and/or consultation fees for Novo Nordisk, Roche, Sobi, Takeda, and CSL Behring. A. Casini has received grants and fees from Octapharma, Sobi, LFB, Takeda, and Novo Nordisk. The institution of R.E.G.S. has received speaker’s fees and/or research grants from Bayer, CSL Behring, Hemab, Novartis, Novo Nordisk, Octapharma, Roche, Sobi, and Takeda. The remaining authors declare no competing financial interests.
Correspondence: Flora Peyvandi, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Via Pace 9, Milan 20122, Italy; email: flora.peyvandi@unimi.it.
References
Author notes
S.M. and R.P. contributed equally to this study.
Data are available upon request from the corresponding author, Flora Peyvandi (flora.peyvandi@unimi.it).