Visual Abstract
Immune effector cells (IECs) include a broad range of immune cells capable of modulating several disease states, including malignant and nonmalignant conditions. The growth in the use of IECs as both investigational and commercially available products requires medical institutions to develop workflows/processes to safely implement and deliver transformative therapy. Adding to the complexity of this therapy are the variety of targets, diseases, sources, and unique toxicities that a patient experiences following IEC therapy. For over 25 years, the Foundation for the Accreditation of Cellular Therapy (FACT) has established a standard for the use of cellular therapy, initially with hematopoietic cell transplantation (HCT), and more recently, with the development of standards to encompass IEC products such as chimeric antigen receptor (CAR)-T cells. To date, IEC therapy has challenged the bandwidth and infrastructure of the institutions offering this therapy. To address these challenges, FACT has established a programmatic framework to improve the delivery of IEC therapy. In this study, we outline the current state of IEC program development, accreditation, and solutions to the challenges that programs face as they expand their application to novel IEC therapy.
Introduction
Cellular therapy (CT) for both malignant and nonmalignant indications has grown in the field of medicine. Genetically engineered immune effector cells (IECs) (for example, chimeric antigen receptors [CARs] and T-cell receptor–modified T cells) and nonengineered IECs (for example, tumor-infiltrating lymphocytes and virus-specific cytotoxic T cells) are widely used as investigational products, with several achieving the US Food and Drug Administration (FDA) approval for hematologic malignancy indications. The scientific rationale, preclinical development, and clinical use of IEC therapy have been extensively reviewed and are not covered in this article. Rather, the focus will be on the challenges institutions face in implementing the expansive CT portfolio due to the number of products, diverse targets, varied indications, and unique side effects of CT. The expansion of IECs to include solid tumors and nonmalignant indications will further complicate the workflows of traditional CT/hematopoietic cell transplantation (CT/HCT) programs, where most IEC therapy is delivered. As IEC therapy grows beyond CT/HCT programs, dissemination of standards used to maintain robust quality in the field of cellular therapy is required. These standards provide a framework for establishing a quality program that spans patient selection, cell collection, treatment, management of unique toxicities, and long-term follow-up, including reporting product-specific sequelae.
To meet this challenge, the Foundation for the Accreditation of Cellular Therapy (FACT) leveraged its expertise and infrastructure to promote quality IEC therapy. Its mission is to improve the quality of both hematopoietic stem cell transplantation and CT through peer-developed standards, education, and accreditation for the benefit of patients. Propelled by the increasing use of IECs in the clinic, FACT has accredited 139 immune effector CT clinical programs in the United States (including their embedded or contracted apheresis and cell-processing facilities) as of 4 April 2023, under either the FACT-JACIE International Standards for Hematopoietic Cellular Therapy Product Collection, Processing, and Administration or the FACT Standards for Immune Effector Cells. These include 138 programs accredited as part of the HCT programs and 1 as a stand-alone IEC program. In this review, we outlined the basic definition of IEC therapy, need for quality oversight, IEC resource use, IEC program structure, need for guidance on donor/recipient selection, product chain of custody/labeling, and developing/maintaining clinical competence for safe and effective administration of IEC products.
Background of the FACT
The FACT is a standard-setting and accreditation organization founded by the American Society for Transplantation and Cellular Therapy (ASTCT) and the International Society of Cell and Gene Therapy (ISCT) in 1995. The mission of the FACT is to improve the quality of HCT and CT through peer-developed standards, education, and institutional accreditation for the benefit of patients. The first edition of the FACT Standards for HCT was published in 1996 based on the merger of the clinical standards developed by the Clinical Affairs Committee of ASTCT and the laboratory standards of the ISCT regulatory affairs committee.1 Since 1999, the Joint Accreditation Committee of ISCT-Europe and the European Society for Blood and Marrow Transplantation (JACIE) have codeveloped these standards, now entitled FACT-JACIE International Standards for Hematopoietic Cellular Therapy Product Collection, Processing, and Administration.2 These comprehensive standards apply to all phases of collection, processing, cryopreservation, storage, and administration of hematopoietic CT, regardless of the tissue source (bone marrow, peripheral blood, cord blood, or other tissue sources). These standards define the infrastructure required for the safe and efficacious collection, processing, and clinical use of hematopoietic cells, including a comprehensive quality management program and ongoing assessment of patient outcomes. These standards include many regulatory requirements from FDA and the directives of the European Union. Although the standards are jointly published, FACT and JACIE maintain separate accreditation programs. The trust and recognition of stakeholders in FACT-accreditation led to subsequent requests for similar standards and accreditation for cord blood banking,2 emerging cellular therapies,3 and, most recently, IECSs.4
Definition of immune effector cells
As noted, the scientific rationale, preclinical development, and clinical use of IEC therapy have been extensively reviewed and will not be covered.5-13 FACT defines an IEC as “a cell that has differentiated into a form capable of modulating or affecting a specific immune response.” This definition includes the broad group of effectors listed in Table 1. Not included are hematopoietic progenitor cells and nucleated immune cells are used as unselected donor lymphocyte infusions and are covered under the FACT-JACIE Hematopoietic Cellular Therapy Standards.1
FACT Standards for IECs
FACT Standards for Immune Effector Cells4 and their accompanying accreditation programs have been created in response to the increasing use, clinical success, and complexity of IEC therapy. FACT developed these standards with collaborators from its founding organizations (ASTCT and ISCT), academic cell-therapy experts, and a representative of the Society for Immunotherapy of Cancer in 2018.1,14 Simultaneously, the FACT Standards unique to IECs were added to the FACT-JACIE Hematopoietic Cellular Therapy Standards (Edition 6, Version 6.1) with the acknowledgment that many transplantation programs are active in clinical trials and the therapeutic use of these products. The scope of the FACT Standards for Immune Effector Cells mirrors those developed for HCT programs and includes the clinical program and collection and processing facilities associated with IEC therapy.
The FACT Standards and accreditation do not outline the requirements for the scientific validity of IEC therapies or cover commercial manufacturing companies; rather, they expect a robust quality/safety program in which the source material is collected/handled and subsequently where final therapies are received, handled, and administered. Adherence to the FACT Standards requires the development of a program infrastructure that encompasses a comprehensive quality management program with provider training in the specific therapies administered and the development of written protocols and/or standard operating procedures (SOPs) on the clinical usage of IECs. These SOPs encompass patient selection, product collection/procurement, product administration, adverse event management, and evaluation and reporting of safety/outcome end points (preferably via the Center for International Blood and Marrow Transplant Research). Essential to the successful implementation of a robust quality management plan is the continuous self-assessment of the effectiveness of SOPs in the safe and effective use of IEC therapy. The standards are applicable wherever IECs are administered and are meant to improve the safety and quality of clinical trials, regulatory approval, commercialization, and reimbursement of IEC therapy, thereby increasing patient access to these life-saving treatments.
IEC therapy—need for quality oversight and accreditation
As previously outlined, the continued growth of the CT field will create new challenges for the safe and effective delivery of IEC therapy. The FACT IEC Standards and Accreditation Program provides educational, instructional, and oversight roles related to program infrastructure, donor and patient care, and the use of IEC therapy. Quality infrastructure centered on the key aspects of safe and efficacious collection, processing, and use of IEC products is paramount. Any programmatic infrastructure related to quality is distinct from the Risk Evaluation and Mitigation Strategies (REMS) program, which focuses on the specific risks of a specific drug/IEC product. Critical to infrastructure development is an independent and ongoing assessment that occurs through the process of FACT accreditation. FACT accreditation is voluntary (although it is required by many cellular-therapy product manufacturers and payers) and based on documented compliance with all current standards. Each edition of the FACT Standards is accompanied by an accreditation manual that includes explanations and examples of ways applicants can meet the standards. FACT accreditation results in demonstrable improvement in program quality1,15 and is thus a prerequisite for cooperative groups for clinical trial participation, requisite for institutional designation as a center of excellence, and for site certification to administer some commercial IEC therapy. It is also used by payers as a criterion for reimbursement. Importantly, as the number of cellular-therapy sponsors/manufacturers grows it is foreseeable the use of FACT accreditation can be used as a litmus test to define which treatment centers have the necessary quality and infrastructure to handle these unique IEC products. Onboarding treatment centers with a multitude of cellular-therapy sponsors is a time-consuming and complex endeavor. FACT accreditation has the potential to alleviate some of the difficulties with that process by identifying treatment centers with the commitment and resources to deliver IEC therapy with the highest quality. Patient access to novel IEC therapy can be increased by reducing this burden to both treatment centers and sponsors/manufacturers. As of 1 March 2023, there are currently 252 CT organizations (193 clinical programs) in 8 countries accredited by FACT and 256 programs accredited by JACIE, with accreditation applications from 35 countries. Table 2 provides an outline of the requirements of the IEC Standards and their importance in providing quality CT. The remainder of this article highlights the common challenges faced by institutional IEC programs and how FACT Standards can improve their ability to deliver quality IEC therapy.
Challenges of IEC programs
Resource utilization
Given the rapid increase in the use of IEC products, clinical centers require an assessment of resources to ensure the safe implementation of IEC therapies. Owing to the nature of IEC therapy, the number of departments and team members involved can be significant. This includes a dedicated team of cellular therapy–trained staff, a blood bank, an apheresis service, a cell-processing laboratory, an adequately equipped pharmacy, and specialty physician consultants who aid in the management of postinfusion toxicities.4 Programmatic assessment of the current staffing levels and expertise is required before the implementation of any IEC therapy. This includes training and/or increasing staffing to identify appropriate patients, coordinating product collection, carrying out product infusion, and ensuring that postinfusion toxicity management aligns with product recommendations. For FDA-approved IEC products, REMS training is often required and will need to be documented by the relevant personnel. Management of postinfusion complications also requires training of consultants and personnel outside the IEC program, including but not limited to staff of the emergency departments, neurology service, and intensive care units. Evaluation of the apheresis service and processing laboratory to meet the product requirements is also required. This may include the need for additional equipment for product collection and storage of the final products, enhanced capabilities to ship and receive cellular products, and sufficient staff to execute this process. Beyond the staff working directly with IEC products, other staff and teams must be trained by the IEC program. This includes staff to track and implement evolving competencies within an IEC program, negotiate and maintain contracts with third-party manufacturers of CT products, obtain financial approval from payors who may be unfamiliar with IEC therapy, coordinate patients’ episodes of care, order, and track products through complex individual manufacturer portals and data managers to handle the increased reporting workload with novel cellular products. In many cases, the home care staff may play an important role in patient care. Most IEC programs will leverage the currently established HCT workflows, although it must be acknowledged that this will likely require expanded staffing. As CT expands beyond current areas, new staff will be needed to support the implementation of new IEC products.
Unique to IEC therapy is the need for inspections and audits by sponsors/manufacturers of IEC programs. Although in some ways IEC therapy is a medical procedure akin to HCT, a major difference is that, from a regulatory perspective, IECs are regulated by the FDA as “drugs,” and are manufactured by academic institutions, commercial entities, or pharmaceutical companies, who serve as the “sponsors.” Because each sponsor must report to the FDA, they each require inspections of the program’s clinical, collection, and processing infrastructure, sometimes including onsite evaluation of the cell-processing facility or other clinical facilities. Each sponsor-mandated inspection temporarily removes critical personnel from their clinical work, and multiple sponsors carrying out inspections of hospitals and clinics for both investigational and commercial IECs have become a significant time and financial burden and interfere with clinical care. Furthermore, conflicting requirements among manufacturers with little bearing on product safety or integrity add time and complexity to training and cause confusion and stress within institutional IEC programs. Sponsors also audit the training of CT staff and compliance with the REMS, adding to the burden of IEC programs. FACT IEC accreditation has the potential to minimize these disruptions and reduce confusion by harmonizing this process as a single accrediting body. This would reduce the burden of inspections for both sponsors and clinical institutions and has been recommended by the 80/20 task force of the American Society for Transplant and Cell Therapy.16
As IEC products expand to novel cell types (eg, allogeneic CARs), new starting materials (eg, tumor specimens from the operating room), and novel indications originating from new commercial sponsors, the ability to deliver quality therapy may be diminished. To reduce complexity, an IEC program can use FACT Standards to meet these challenges in a coordinated and logical manner to promote the rapid implementation of new therapies while maximizing patient safety and access.
IEC program structure
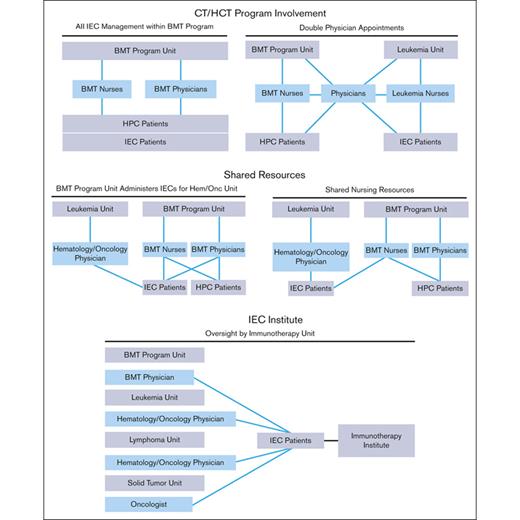
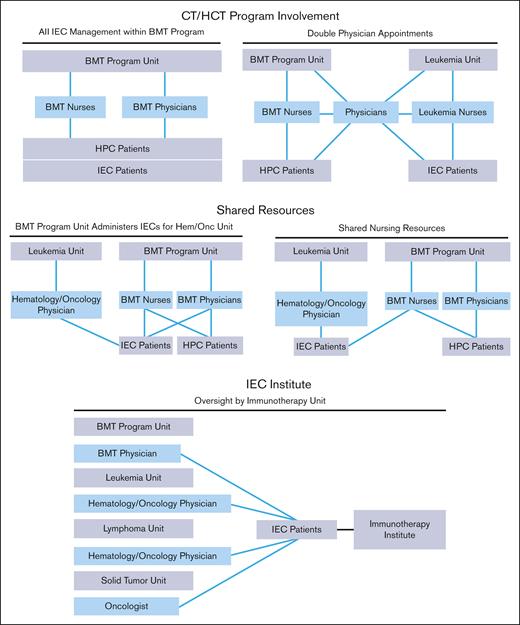
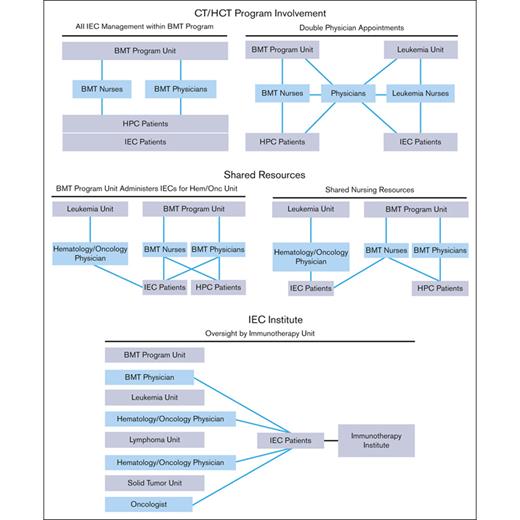
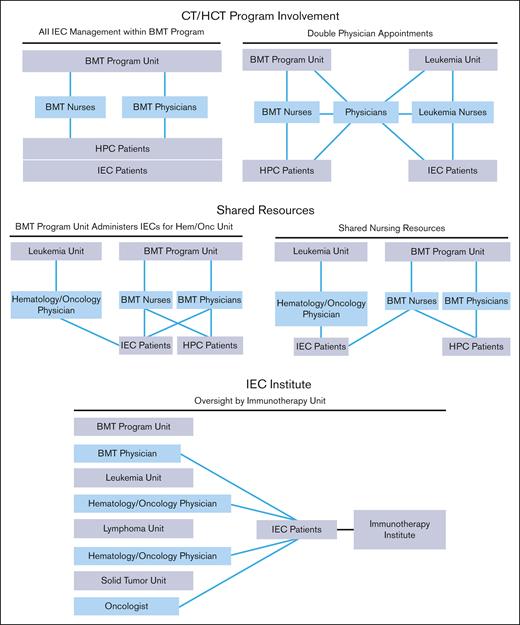
The structure of an IEC program depends on institutional characteristics such as leadership, specialties, facilities, and support services. The diverse indications, cell sources, and manufacturing methods of IECs have resulted in complex patient experiences that span different areas of an institution. Several organizational models are used by IEC programs, all of which can be effective with appropriate lines of communication and defined roles and responsibilities. Regardless of the program structure, inspectors will look for consistently applied quality management activities, procedures, and training throughout the program, seeking FACT accreditation. The following program structures have been successfully developed (Figure 1):
IEC/HCT combined program: At the time of writing, most IEC programs are part of an IEC/HCT combined program. In this model, physicians and nurses within a single programmatic unit manage patients with both HCT and IEC, whereas physicians and nurses within different programmatic units may share clinical responsibilities for patients with both HCT and IEC. In most cases, HCT and IEC programs have patients with common diagnoses (eg, hematologic malignancies) who are cared for by physicians with similar disease expertise, enabling the use of shared resources, such as scheduling, financial approvals, cell collection/cell processing, treatment/management of postinfusion complications, inpatient/outpatient facilities, and infrastructure to track patient outcomes.
Shared IEC and HCT program: Some models may share resources, such as nursing staff, space, and quality management resources, without being integrated. In this overlap model, patients with IEC are given cell products on a shared IEC/HCT unit and are cared for by IEC/HCT-trained nurses, but the physicians for patients with IEC and HCT are not integrated.
Stand-alone IEC program: In this model, the IEC program may stand alone, and IECs are administered on several different units; however, the directors of the program oversee IEC activity across all units. The quality management plan, staff training, and protocols are consistent among units that offer IEC therapies.
IEC program structures. BMT, bone marrow transplant; HPC, hematopoeic progenitor cell.
IEC program structures. BMT, bone marrow transplant; HPC, hematopoeic progenitor cell.
Patient selection
Crucial to a quality IEC program is the development of guidance and SOPs related to patients with IEC selection. In the setting of investigational trials, patient selection will be governed by the protocol inclusion and exclusion criteria. However, as more IEC products gain commercial approval, a more robust method of patient evaluation is required to avoid inappropriate patient selection. This may include accurately assessing target antigens (eg, by flow cytometry or immunohistochemistry) to match product targets, guidelines on collection suitability (eg, adequate lymphocyte counts), and general evaluation of patients’ performance status and comorbidities. The development of a robust workflow to identify candidates for IEC therapy is imperative because the unique toxicities of IEC therapy can result in significant morbidity following treatment. Once institutional criteria are established, appropriate patient selection can be ensured through regularly scheduled case reviews of potential patients (eg, weekly rounds of new patients). When a patient fails to meet predefined criteria, establishing methods to proceed with additional safeguards can effectively address patient suitability and provide access to IEC therapy. For example, if a patient has reduced cardiac function, 1 strategy would be to implement early anticytokine/cytokine release syndrome toxicity therapy to reduce any morbidity that the patient may face. Predefining guidance on patient selection will permit the optimal application and use of cell therapies.
Product procurement, chain of custody, and product labeling
Fundamental to a quality IEC program is the development of rigorous workflows that ensure the proper procurement of IEC products. This includes a chain of custody and product labeling procedures that ensures that IEC products are not misidentified, misguided, or misused. The IEC program should ensure the traceability of CT products and that the chain of custody for all cellular therapy products manufactured locally and/or by a third party is maintained. Chains of identity and custody are critically important given the potential involvement of separate entities and multiple transfers of cells intended for a specific patient. This process must be designed to ensure that the right CT product is administered to the right person at the appropriate time. In general, no analytical testing is performed on the final product to identify a mixture of products, and failure within the chain of identity or custody processes could lead to serious health risks to the patient. FACT requires quality systems that enable tracking and tracing from the cell donor (including autologous patients) to the final administration or distribution of the product, verification of the product, and patient identity at multiple steps. This includes strict labeling controls such as the use of ISBT 128 coding and labeling. As new sites of product procurement (eg, operating room/pathology laboratory for tumor-infiltrating lymphocytes) are added, the implementation of workflows to other areas of the institution will be needed and will challenge the currently established workflows within an organization.
Clinical expertise and maintaining competency
The FACT Standards require the identification of a clinical program director and attending physicians with appropriate experience and training in IEC therapy. Training and competency should be demonstrated for indications of cell therapy, patient selection, product administration, and clinical management, including the identification/management of unique toxicities that occur following cellular therapies. These unique toxicities can appear days after cell infusion and evolve rapidly. The training of health care teams to identify and manage potential toxicities such as cytokine release syndrome, immune effector cell associated neurotoxicity, and the effects of preconditioning/lymphodepleting chemotherapy are critical to the safe delivery of IEC products. The availability and knowledge of when to administer specialized drugs, such as anticytokine monoclonal antibodies (eg, tocilizumab or anakinra) or corticosteroids, are required. Even in circumstances where IEC product toxicity is minimal or rare, it is essential to be prepared and equipped to manage these potential unique toxicities. The development and refinement of SOPs for the clinical management of IEC products will depend on the types of IEC products available to clinicians within a program. As new targets, enhanced activity, and the development of “off-the-shelf” allogeneic products have emerged, FACT IEC standards and program guidance must evolve to manage any unforeseen toxicity. IEC programs should regularly review controlled documents (including guidelines and SOPs), obtain continuous education, and monitor patient outcomes to ensure that their criteria for success match current IEC therapies.
Conclusion
IEC therapy has been transformative in providing clinically meaningful benefits to patients across a spectrum of indications and diseases. The excitement and potential of IEC therapy have spurred its expansion to various targets, diseases, and cell sources. The unique toxicities associated with institutional and product-specific management have increased the complexity of delivering safe and effective IEC products. In response, the FACT has developed standards that provide a framework for program development centered on quality and continual self-assessment to improve patient outcomes following IEC product administration. The development of standards related to patient selection, product collection/procurement, product administration, adverse event management, and the evaluation and reporting of safety/outcome end points is the basis of a quality IEC program. We addressed some of the common issues faced by IEC programs, including the need to manage resources, a proper program structure, and the need for ongoing competency assessment. Through the FACT IEC Standards and Accreditation Program, institutions can develop a quality IEC program that provides safe and effective products for patients with exceptional outcomes.
Acknowledgments
The authors thank Joseph Olechnowicz, Editor, Memorial Sloan Kettering Cancer Center, Department of Pediatrics, for editorial assistance.
K.J.C. acknowledges the National Institutes of Health Cancer Center support grant P30 CA008748.
Authorship
Contribution: All authors made substantial contributions to the conception and design of this work, assisted in drafting or substantive revision of the manuscript and approved the submitted version, and agree to be held accountable for all aspects of this work.
Conflict-of-interest disclosure: K.J.C. serves on the advisory board/received consultancy fees from Novartis, Atara Biotherapeutics, and Turn Bio, and receives research funding from Novartis, Celegene, and Cellectis. Y.-M.H. serves on the advisory board for and received research funding from Avalon GloboCare Corp. H.E.H. is a cofounder with equity in Allovir and Marker Therapeutics; has share options in CoRegen and Fresh Wind Biotherapies; has served on advisory boards for GSK, Tessa Therapeutics, and Fresh Wind Biotherapies; and receives research support from Tessa Therapeutics and Athenex. M.V.M. is an inventor of patents related to adoptive cell therapies, held by Massachusetts General Hospital (some licensed to Promab) and University of Pennsylvania (some licensed to Novartis); receives grant/research support from Kite Pharma; is a consultant for multiple companies involved in cell therapies; holds equity in 2seventy Bio, Century Therapeutics, Neximmune, Oncternal, and tumor-infiltrating lymphocytes 2; and is a member of board of directors for 2seventy Bio. D.M. has served on the advisory boards for Bristol Myers Squibb (BMS), Caribou Biosciences, Inc, Genentech, Incyte, Juno Therapeutics, Kite, Lilly, Mustang Bio, Novartis, and Umoja; has the rights to royalties from Fred Hutch for patents licensed to Juno Therapeutics/BMS; and has received research support from Kite Pharma, Juno Therapeutics, BMS, and Legend Biotech; and has stock options in A2 Biotherapeutics and Navan Technologies. P.M. serves on the advisory board and provides consulting for bluebird bio, BMS, Celgene, Fate Therapeutics, Janssen, Karyopharm, Magenta Therapeutics, Sanofi, and Takeda, and receives honoraria from bluebird bio, BMS, Celgene, Fate Therapeutics, Janssen, Karyopharm, Magenta Therapeutics, Sanofi, and Takeda. D.P. reports a consulting or advisory role for Novartis, Kite/Gilead, Incyte, Gerson Lehrman Group, Janssen (Johnson & Johnson), DeCART, BMS, bluebird bio, Angiocrine, Mirror Biologics, Capstan Therapeutics, and Instill Bio; has stock and other ownership interests in Genentech and Roche (spouse former employment); receives research funding from Novartis; reports patents, royalties, and other intellectual property from Novartis and Tmunity; and is a patent inventor for the use of CAR-T cells in CD19+ malignancies. E.J.S. serves on advisory boards for Adaptimmune, Axio, Celaid, FibroBiologics, Navan, Novartis, NY Blood Center, Partner Therapeutics, Mesoblast, and Bayer Pharmaceuticals, and has licensing agreements with Syena, Affimed, and Takeda. B.W. reports consultancy with Guidepoint Global, and serves on the advisory board for MorphoSys and ADC Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Kevin J. Curran, Memorial Sloan Kettering Cancer Center, Department of Pediatrics, 1275 York Ave, New York, NY 10065; email: currank@mskcc.org.