TO THE EDITOR:
Chimeric antigen receptor (CAR) T-cell (CAR-T) therapies genetically modify a person’s own T cells to bind to antigens expressed on cancer cells.1,2 CAR-T has generated sizable enthusiasm because of early reports of dramatic and sustained response.3 Since 2017, 6 CAR-T products have been approved by the Food and Drug Administration (FDA), and other indications for a wide variety of tumors, including solid cancers, are actively being tested.4-7
CD19 CARs have been associated with high response rates and a fraction of patients experiencing durable complete remissions.8,9 B-cell maturation antigen CARs have hitherto been associated with high response rates but concerningly high rates of eventual relapse.
For these reasons, and consistent with prior works, which have examined genome driven therapies, checkpoint inhibitors, and cytotoxic drugs,10-13 we sought to estimate the percentage of patients in the United States with advanced or metastatic cancer who may be eligible for and respond to CAR-T therapies.
We considered a person as being eligible for CAR-T therapy if they had the tumor type and notable inclusion criteria for the drug approval. In accordance with 45 Code of Federal Regulations section 46.102(f), this study was not submitted for institutional review board approval because it involved publicly available data.
In order to estimate the number of persons in the United States with advanced or metastatic cancer who were eligible, according to the approval indications, we used death data for the respective tumor type from the American Cancer Society’s Cancer Facts and Figures.14 These data were used as a stand-in for eligibility because these therapies are often administered in later lines when other frontline drugs and therapies have been unsuccessfully used. We abstracted data corresponding to the years the individual malignancy types had a CAR-T therapy approval, through 31 March 2023.
Because several indications were approved for a more specific tumor type than that used for death data, we searched the peer-reviewed literature for specific prevalence estimates for those tumor types. For eligibility, we assumed that 20% of patients with multiple myeloma progressed to fourth line or later.15 We assumed that 22% of non-Hodgkin lymphoma (NHL) was follicular lymphoma;16 3% of NHL was mantle cell lymphoma;16 and 75% of patients with NHL had large B-cell lymphoma (after subtracting follicular and mantle cell lymphomas). For B-cell precursor acute lymphoblastic leukemia, we assumed that 75% of acute lymphoblastic leukemia cases were of the B-cell lineage.17,18
For all CAR-T therapies approved by the FDA, we used package inserts to identify trial data, indication, and the reported overall response rate. Response rates were based on complete and partial responses, including complete remission with or without complete hematologic recovery.
We divided the number of cancer-specific deaths for which there was an FDA-approved CAR-T therapy by the total number of all cancer deaths to estimate the percentage of people who were eligible for CAR-T therapy.
For each cancer type, the estimated number of people eligible was multiplied by the response rate. This provided an estimate of the cancer-specific benefit. Because there were several CAR-T therapies for NHL and acute lymphoblastic leukemia indications, we used the highest calculated response rate to provide the best-case estimate. The estimated cancer-specific benefit for all indications were totaled for each year a therapy had an approval. The sum of total cancer-specific benefits for each year was divided by the total number of people who died from all the cancers for which there was a CAR-T therapy approved. This provided an overall estimate of response to CAR-T therapies.
We calculated frequencies of trial characteristics and 95% confidence intervals (CIs) for the estimated percentage of eligibility and response using R statistical software, version 4.2.1 (R Project for Statistical Computing). All other calculations were performed using Excel (Microsoft Corporation).
We found 6 CAR-T therapies that were approved for 5 tumor types, for a total of 13 approval indications (Table 1).
Three (23.1%) approvals were via the accelerated pathway. Two (15.4%) approvals were based on randomized data. The median number of participants the intervention arm enrolled and underwent leukapheresis in the intervention arm was 108 (range, 71-299). The median reported response rate for all trials leading to a CAR-T therapy approval was 66.4% (range, 25.5%-90.9%).
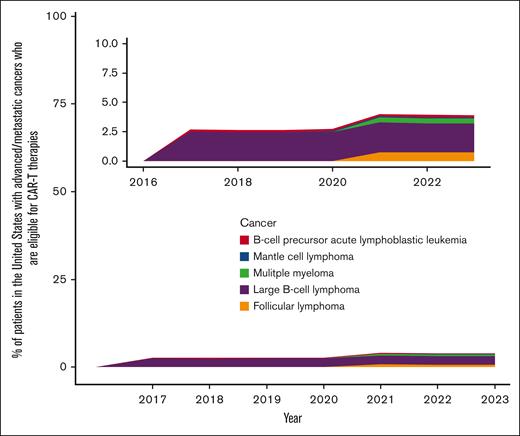
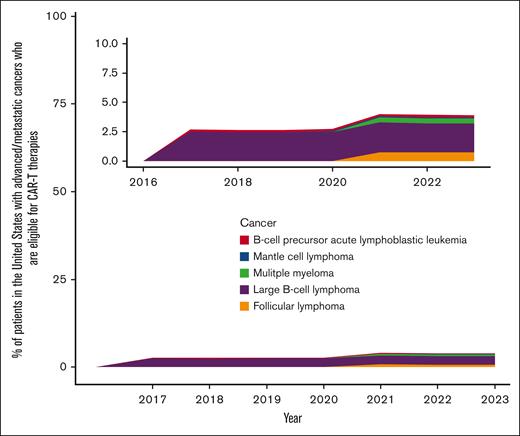
The percentage of people with advanced or metastatic cancers who were eligible for CAR-T therapies was estimated to be 2.7% (95% CI, 2.6%-2.7%) in 2017 and 3.9% (95% CI, 3.8%-3.9%) in 2023 (Figure 1). In 2023, the eligibility was highest for patients with large B-cell lymphoma (2.5%; 95% CI, 2.4%-2.5%) and lowest for mantle cell lymphoma (0.1%; 95% CI, 0.1%-0.1%).
Percentage estimates of patient eligibility for CAR-T therapies that have received US FDA approval.
Percentage estimates of patient eligibility for CAR-T therapies that have received US FDA approval.
Using the reported response rates in the FDA package inserts, a more optimal situation when based on per-protocol analysis, the percentage of people with advanced or metastatic cancer who had the potential to respond to CAR-T therapies was 2.0% (95% CI, 1.9%-2.0%) in 2017 and 3.4% (95% CI, 3.4%-3.5%) in 2023. In 2023, the response was highest for patients with large B-cell lymphoma (2.1%; 95% CI, 2.1%-2.2%) and lowest for mantle cell lymphoma (0.09%; 95% CI, 0.08%-0.09%).
We estimated the percentage of patients in the United States with advanced or metastatic cancer who may be eligible for and respond to CAR-T therapies to be ∼3.9% and 3.4%, respectively. These estimates are likely to be an upper bound for several reasons: (1) some patients will die before having opportunity to receive these therapies, (2) cost and logistics may be a barrier to receiving therapies, and (3) performance status and age may preclude these therapies. Prior empirical work has shown that <1 in 5 patients likely receive therapies for which they are eligible.35
The potential response was higher for CAR-T therapies (median of 66%) than for other therapy types (50% for cytotoxic12 and 19% for genome informed therapy10); however, currently, all but 2 of the registration trials are uncontrolled. Both randomized trials showed improvements in overall survival (lisocabtagene: 12-month survival was 79.1% vs 64.2%; P < .001; axicabtagene: 4-year overall survival was 54.6% vs 46.0%; P = .03)23,36 Moreover, study outcomes for diffuse large B cell lymphoma trials may be better than they would be in real-world settings because they include patients who are at lower risk (excluding patients who need bridging therapy and higher Eastern Cooperative Oncology Group performance status),37 although the ZUMA-7 trial did allow bridging therapy. For multiple myeloma, real-world data indicate that responses are similar, whereas overall survival is better for trial participants.38
Our estimates are limited because we used deaths as a stand-in for people who would be eligible. These data indicate who would likely have died without CAR-T therapy. Our estimates provide a current best-case scenario, and actual eligibility is likely much lower because many people will not receive them, owing to health or financial reasons. Future approvals in earlier lines would increase eligibility, but randomized studies would need to be done to ensure that these therapies improve patient outcomes, cost effectively.
We found that although the response rate to CAR-T therapy was high, the overall eligibility and potential response to these therapies, as a percentage of patients with advanced or metastatic cancer, is 3.9% and 3.4%, respectively. Most CAR-T approvals are supported by single-arm trials using surrogate outcomes. Adjusting for patients who do not receive these products lowers CAR-T response rates by 3%. Between 2017 and 2023, CAR-T eligibility has grown at an approximate average of 0.6% patients per year. Future research may better define real-life eligibility and response.
Acknowledgment: This study was funded by Arnold Ventures.
Contribution: A.H. and V.P. conceptualized the study design; A.H. abstracted and analyzed data, and wrote the first draft of the manuscript; V.P. and T.B.H. reviewed data; T.B.H. and V.P. reviewed the manuscript draft; and all authors approved the final draft.
Conflict-of-interest disclosure: V.P. acquired research funding from Arnold Ventures; reports royalties from Johns Hopkins Press, Medscape, and MedPage; honoraria from Grand Rounds/lectures from universities, medical centers, nonprofits, and professional societies; provided consultancy for UnitedHealthcare and OptumRX; and has other participation in Plenary Session podcast, which has backing via Patreon, YouTube, and Substack. The remaining authors declare no competing financial interests.
Correspondence: Alyson Haslam, Department of Epidemiology and Biostatistics, University of California San Francisco Medical Center, UCSF Mission Bay Campus, Mission Hall: Global Health & Clinical Sciences Bldg, 550 16th St, 2nd Floor, San Francisco, CA 94158; email: alyson.haslam@ucsf.edu.
References
Author notes
The data that support the findings of this study are available upon reasonable request from the corresponding author, Alyson Haslam (alyson.haslam@ucsf.edu).