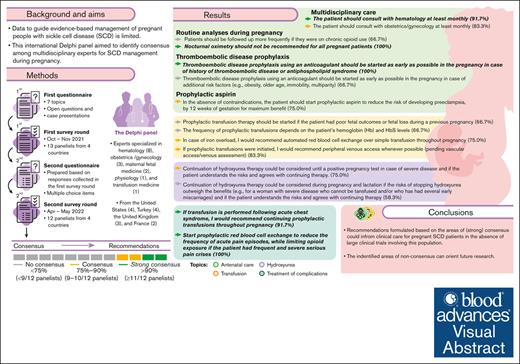
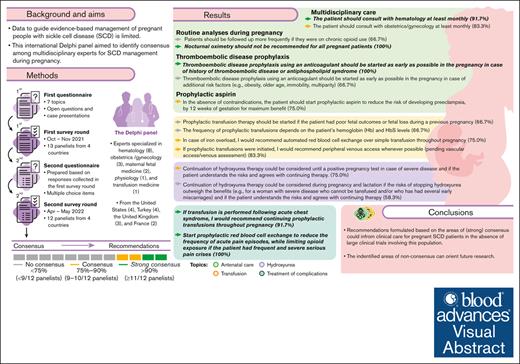
This international Delphi panel of 12 multidisciplinary experts explored 7 topics related to SCD management during pregnancy.
Based on consensus reached in several areas, recommendations were formulated to inform clinical care for pregnant patients with SCD.
Visual Abstract
Data to guide evidence-based management of pregnant people with sickle cell disease (SCD) are limited. This international Delphi panel aimed to identify consensus among multidisciplinary experts for SCD management during pregnancy. The 2-round Delphi process used questionnaires exploring 7 topics (antenatal care, hydroxyurea use, transfusion, prevention of complications, treatment of complications, delivery and follow-up, and bottlenecks and knowledge gaps) developed by a steering committee. Thirteen panelists (hematologists, physiologists, obstetricians, maternal fetal medicine, and transfusion medicine physicians) from the United States, the United Kingdom, Turkey, and France completed the first survey; 12 panelists completed the second round. Anonymized responses were collected and summarized by a contract research organization (Akkodis Belgium). Consensus and strong consensus were predefined as 75% to 90% (9-10 of 12) and >90% (≥11 of 12) of panelists, respectively, agreeing or disagreeing on a response to a predefined clinical scenario or statement. In several areas of SCD management, consensus was achieved: experts recommended performing at least monthly multidisciplinary antenatal follow-up, administering prophylactic aspirin for preeclampsia prevention between gestational weeks 12 and 36, initiating prophylactic transfusion therapy in certain cases, or choosing automated red blood cell exchange over other transfusion methods for patients with iron overload or severe acute chest syndrome. No consensus was reached on several topics including the prophylactic aspirin dose, indications for starting infection prophylaxis, routine use of prophylactic transfusions, or use of prophylactic transfusions for preventing fetal complications. These recommendations could inform clinical care for patients with SCD who are pregnant in the absence of large clinical trials involving this population; the identified knowledge gaps can orient future research.
Introduction
Over the past decades, important advances have been made in the field of therapeutic options for sickle cell disease (SCD), the world’s most common genetic blood disorder.1 Disease-modifying treatments, including drugs like hydroxyurea or red blood cell (RBC) transfusion (ie, simple top-up transfusion [ST], manual RBC exchange [mRBCx, consisting of ST and phlebotomy], or automated RBC exchange [aRBCx]), play a major role in preventing complications.1 Early detection and infection prevention have led to a decrease in childhood mortality caused by SCD, which is now considered a chronic disease in high-income countries.2 This, in turn, shifts the burden of disease more toward the adult population, including pregnant individuals. Compared to their healthy counterparts, pregnant people with SCD have worse maternal and fetal outcomes, with higher rates of preeclampsia and eclampsia, premature birth, and intrauterine growth retardation.3,4 In addition, the risk of SCD-related complications like acute pain episodes requiring hospitalization or acute chest syndrome (ACS) increases during pregnancy.5
Therapeutic options for SCD management (including SCD-modifying therapy and supportive interventions for pain control) are limited for pregnant people.6,7 Use of hydroxyurea is not recommended during pregnancy because of a potentially increased risk of miscarriage and stillbirth, and concerns about teratogenic effects.8-10 Opioids commonly used for pain management can cause neonatal abstinence syndrome and other negative outcomes in the newborns when administered during pregnancy.11 To date, use of recently approved disease-modifying therapies, such as L-glutamine, crizanlizumab, and voxelotor,12 has not been investigated during pregnancy. With regard to prophylactic transfusion therapy, the strongest data supporting its use for pregnant people with SCD is a meta-analysis of 1291 individuals; the aforementioned study showed that prophylactic transfusions reduced the frequency of acute pain episodes, maternal and fetal/neonatal mortality, pulmonary embolism, pyelonephritis, and preterm birth.13 Several recommendations and guidelines of professional societies include criteria for initiating prophylactic transfusions for pregnant people with SCD.7,14,15 Nevertheless, limited high-quality data exist currently to orient evidence-based clinical decision making for SCD management during pregnancy, as highlighted by professional societies and national guidelines addressing this topic.7,14-16
Several barriers stand in the way of advancing research in pregnant people with SCD. Most individuals with SCD live in low-resource settings, in which access to safe blood transfusions is limited.17,18 In resource-rich countries, SCD is classified as a rare disease,19 and individuals with SCD often face significant socioeconomic barriers to medical care,20 especially in countries without a universal healthcare system. This makes the completion of large, multicenter, patient-oriented studies challenging. In addition, pregnant individuals are often excluded from randomized controlled trials because of concerns about maternal and fetal risks,21 which leads to a paucity of high-quality data to guide clinical care of this population.
In this study, the Delphi methodology was used to obtain expert consensus on several areas of SCD management during pregnancy. The Delphi process represents a method of developing consensual guidance on best practice, especially in areas in which clinical data are scarce.22,23 It consists of multiple iterative stages designed to guide the transformation of individual opinions into a group consensus by using controlled feedback.22 Briefly, a Delphi panel comprising experts provides answers to a set of items anonymously, to avoid peer pressure; their collective input is then used to design the subsequent rounds of the survey.
With this Delphi panel on SCD management during pregnancy, we aimed to assess leading experts’ opinions on the treatment and prevention of SCD-related complications in pregnant individuals, and to develop consensus-based management recommendations to serve as guidance for healthcare professionals and organizations.
Methods
Experts
The Delphi process was driven by a steering committee comprised of 3 lead hematology experts (D.S., I.K., and J.H.) with extensive experience in the management of SCD, who were selected based on their publication record. The steering committee identified 21 physicians with expertise in the management of SCD from the United States, the United Kingdom, India, Belgium, Turkey, and France, who were invited to participate in the Delphi survey. Ten of these experts agreed to participate in the first round of the survey, in addition to the 3 steering committee members. This was a geographically diverse group of experts, specialized in hematology (8), obstetrics and gynecology (OB/GYN, 3), maternal fetal medicine (2), physiology (1), and transfusion medicine (1), who were from the United States (4), Turkey (4), the United Kingdom (3), and France (2). One expert (French hematologist) excused herself from participating in the second round, leaving 12 specialists.
The Delphi process
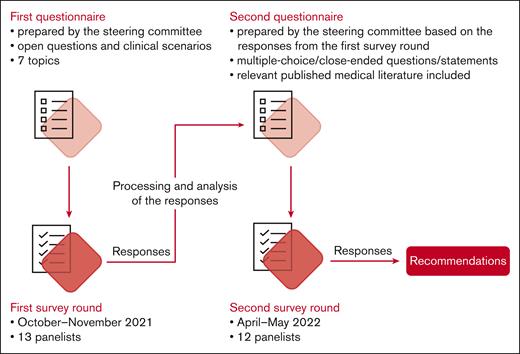
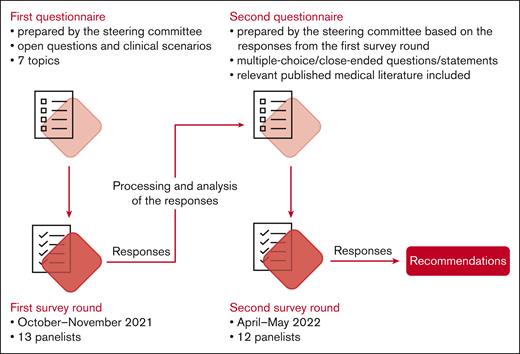
The Delphi survey included 2 iterations (Figure 1). Items for both iterations were elaborated by the steering committee, in collaboration with a life sciences contract research organization (Akkodis Belgium). Clinical scenarios and statements were developed using data obtained from a systematic literature review conducted previously by Terumo Blood and Cell Technologies, with input from the 3 lead experts (results not published), complemented with input from the steering committee members in areas with limited coverage in published data.
Panelists received digital questionnaires created using Jotform (Jotform Inc, San Francisco, CA); they were instructed to respond assuming that no resource constraints existed. The anonymized responses were collected and summarized at Akkodis Belgium.
Items in the first questionnaire were grouped into 7 topics (antenatal care, hydroxyurea use, transfusion, prevention of complications, treatment of complications, delivery and follow-up, and bottlenecks and knowledge gaps) and consisted of a combination between clinical scenarios and open-ended questions (supplemental File 1). Items in the second questionnaire consisted of multiple-choice, close-ended questions or statements formulated based on the responses from the first round (supplemental File 2). For this round of the survey, relevant published medical literature was also provided to the panelists. Topics and clinical scenarios were kept as consistent as possible between rounds. The panelists had the opportunity to discuss the survey results during online meetings.
In the second round of the Delphi survey, consensus was predefined as ≥75% of panelists (≥9 of 12) agreeing/disagreeing on the same answer.24 An agreement/disagreement between >90% of panelists (≥11 of 12) was considered strong consensus; if <75% of panelists (<9 of 12) selected the same answer, no consensus was reached.
Results
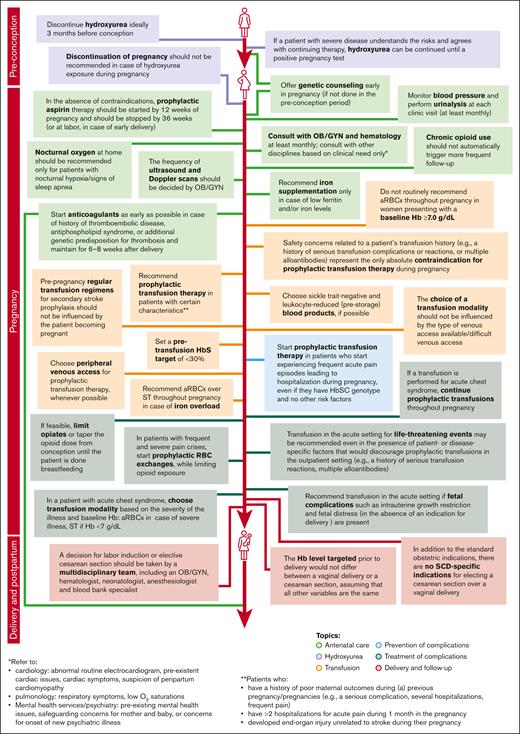
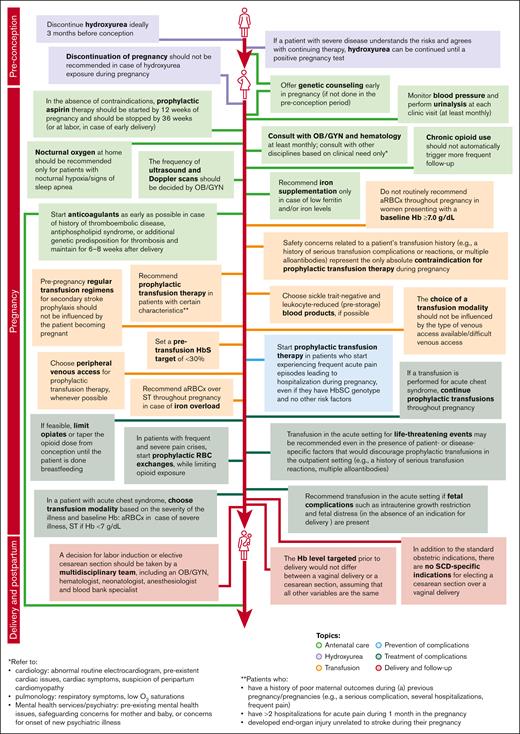
In both rounds of the Delphi survey, 100% of the panelists provided answers (13 of 13 in the first round, and 12 of 12 in the second round). Consensus reached during the second iteration guided the development of recommendations for management by healthcare professionals of pregnant individuals with SCD (Figure 2).
Recommendations for SCD management during pregnancy elaborated based on consensus reached during the second round of Delphi survey.
Recommendations for SCD management during pregnancy elaborated based on consensus reached during the second round of Delphi survey.
The areas of agreement/disagreement between panelists after the second survey round are described hereafter for each topic.
Antenatal care
Most panelists agreed that pregnant individuals with homozygous sickle hemoglobin (hemoglobin SS [HbSS]) genotype and previously mild disease should consult with obstetrics and gynecology (10 of 12, 83.3%) and hematology specialists (11 of 12, 91.7%) at least monthly, and with other disciplines (eg, cardiology, pulmonology, interventional radiology, perinatology and neonatal specialist, and mental health services) based on clinical need only (10 of 12, 83.3%). Consensus was also reached regarding indications warranting referral to cardiology, pulmonology, and mental health services/psychiatry (Table 1). All panelists agreed that if not done in the period before conception, genetic counseling should be offered early in pregnancy. All responders agreed that OB/GYN specialists should determine the frequency of ultrasound and Doppler scans for fetal growth monitoring. Although over half of the panelists (8 of 12, 66.7%) believed that pregnant patients with the HbSS genotype should be followed-up more frequently if they were on chronic opioid use, consensus was not reached.
Panelists agreed that blood pressure monitoring and urinalysis should be performed at least monthly (11 of 12, 91.7%). Although >50% of the panelists (8 of 12, 66.7%) believed OB/GYN specialists should provide this monitoring, others believed that it should be done by a multidisciplinary team or in collaboration with a hematologist; consensus was not reached.
Panelists were aligned on starting thromboembolic disease prophylaxis using an anticoagulant as early as possible in the pregnancy for patients with several additional thrombotic risk factors besides SCD (history of thromboembolic disease, additional genetic predisposition for thrombosis, or antiphospholipid syndrome; ≥9 of 12, ≥75.0%; Table 1). There was no consensus on when prophylactic anticoagulants should be started in the absence of these risk factors, but most panelists (9 of 12, 75.0%) agreed that if started during pregnancy for primary prevention, anticoagulants should be administered up to 6 to 8 weeks after delivery.
Panelists were not aligned on recommending infection prophylaxis during pregnancy: half of the panelists would not routinely start infection prophylaxis using an antibiotic for individuals with HbSS genotype and mild disease.
There was a strong consensus (11 of 12, 91.7%) that iron supplementation during pregnancy would only be needed if the patient had low ferritin and/or iron levels.
Most panelists (10 of 12, 83.3%) would not recommend nocturnal oxygen at home as standard care throughout the pregnancy. However, they would order nocturnal oximetry for pregnant individuals with symptoms of nocturnal hypoxia/sleep apnea (10 of 12, 83.3%), and all panelists agreed that patients with confirmed nocturnal hypoxia should receive nocturnal oxygen at home. All panelists would refer patients found to be hypoxic at rest to a pulmonologist for evaluation.
There was a consensus (9 of 12, 75.0%) that in the absence of contraindications, prophylactic aspirin therapy should be started to reduce the patient’s risk of developing preeclampsia. Most panelists agreed that prophylactic aspirin should be started by 12 weeks of gestation for maximum benefit (9 of 12, 75.0%) and stopped by 36 weeks of gestation (or at labor in case of early delivery; 11 of 12, 91.7%). However, there was no consensus regarding the dose to be used (Table 1), with 2 panelists mentioning that they would defer this decision to OB/GYN specialists.
Hydroxyurea
All 12 panelists agreed that in ideal circumstances, hydroxyurea therapy should be discontinued at least 3 months before conception, to minimize a possible effect on oocyte quality; patients should not take hydroxyurea during pregnancy and until after breastfeeding, to minimize the risk of teratogenicity and newborn exposure. Most panelists (9 of 12, 75.0%) agreed that continuing hydroxyurea therapy could be considered, in case of severe disease, until a positive pregnancy test, if patients understand the risks and agree with this approach. However, only 7 of 12 (58.3%) panelists agreed that hydroxyurea therapy could be continued during pregnancy and lactation if the risks of stopping hydroxyurea therapy outweighed the benefits (eg, for patients with severe disease who cannot receive transfusion and/or who have had several early miscarriages). Most panelists (11 of 12; 91.7%) would not recommend discontinuation of pregnancy in case of hydroxyurea exposure but they would inform the patient and their family about the potential risks.
Transfusion
Half of the panelists would recommend starting regular transfusions immediately after stopping hydroxyurea therapy; consensus was not reached (Table 2). However, panelists who remained neutral or disagreed would recommend transfusions in case of SCD-related complications. More than half of the panelists (7 of 12, 58.3%) disagreed with the use of routine prophylactic transfusions in all pregnant individuals with SCD, but consensus was not reached. Most panelists (10 of 12, 83.3%) would recommend prophylactic transfusion therapy for patients with >2 hospitalizations for acute pain during 1 month in their pregnancy. There was strong alignment (11 of 12, 91.7%) on starting prophylactic transfusion therapy for patients with a history of poor maternal outcomes during previous pregnancies (eg, several hospitalizations or significant pain). Although half of the panelists considered primary and secondary stroke prevention as the only absolute indication for prophylactic transfusion therapy for pregnant individuals with SCD, consensus (9 of 12, 75.0%) was reached on starting prophylactic transfusion therapy if the patient developed end-organ injury unrelated to stroke during pregnancy (eg, pulmonary hypertension, and acute or chronic kidney disease). Consensus was not reached on starting prophylactic transfusion therapy for patients with poor fetal outcomes or fetal loss during previous pregnancies (Table 2), although 3 of the panelists who neither agreed nor disagreed with this statement would consider prophylactic transfusion therapy for fetal losses suspected to result from SCD-related complications. Most panelists (11 of 12, 91.7%) considered safety concerns related to the pregnant patient’s transfusion history (eg, a history of serious transfusion complications or reactions, or multiple alloantibodies) as the only absolute contraindication for prophylactic transfusion therapy.
Panelists were not aligned on the Hb and hematocrit levels to target when recommending prophylactic transfusion therapy for a pregnant patient with SCD (Table 2).
Consensus (9 of 12, 75.0%) was reached on a pretransfusion target of <30% (HbS), although 2 panelists indicated that they would also consider other factors (eg, the indication or the frequency of transfusions accepted by the patient).
Panelists were not aligned on what methods they would recommend for preventing alloimmunization (Table 2). However, all experts would recommend at least 1 preventive measure, including phenotypic matching for Rh and Kell antigens, more extended RBC phenotyping, or RBC genotyping. Although >50% of the panelists (8 of 12, 66.7%) would not choose aRBCx over ST and mRBCx (modified/partial) to prevent alloimmunization (presuming the same method of RBC phenotyping/genotyping), a consensus was not reached.
Consensus was reached on recommending sickle trait–negative (10 of 12, 83.3%) and leukocyte-reduced (pre-storage) blood products (9 of 12, 75.0%) for pregnant individuals with SCD.
For prophylactic transfusions, most panelists (10 of 12, 83.3%) would recommend peripheral venous access whenever possible. Consensus (9 of 12, 75.0%) was also reached that even if venous access is difficult, the same transfusion modality would be recommended, using different access; none of the panelists would change their decision to recommend transfusion in this case.
Most panelists (9 of 12, 75.0%) believed that pregnancy would not influence the choice of transfusion modality (ie, ST, mRBCx [modified/partial], or aRBCx).
Prevention of complications
Most panelists (9 of 12, 75.0%) would start prophylactic transfusion therapy for patients with heterozygous HbS (HbSC) genotype who started experiencing frequent acute pain episodes leading to hospitalization during their pregnancy, even in the absence of other risk factors. Although consensus was not reached, for >50% of the panelists (7 of 12, 58.3%), a patient’s genotype (HbSC vs HbSS) would not influence their decision to start prophylactic transfusions to prevent SCD-related complications, assuming all other disease- and pregnancy-related factors are the same.
Treatment of complications
Consensus was not reached regarding the criteria (including the Hb cut-off level) to be considered for performing a transfusion for a pregnant patient with SCD and progressively worsening asymptomatic anemia (Table 3), although all panelists would recommend transfusions in ≥1 of the proposed scenarios. Transfusion would be recommended by half of the panelists if the patient’s Hb level dropped to <7 g/dL, and by over half of the panelists (8 of 12, 66.7%) if the patient’s Hb level dropped to <4 g/dL, or if the patient became symptomatic. Panelists not selecting the 4 g/dL–Hb threshold preferred to already recommend transfusion at a higher Hb level. Over half of the panelists (8 of 12, 66.7%) would not recommend transfusion after a single uncomplicated pain episode.
All panelists agreed that for pregnant patients with ACS, the choice of transfusion modality should consider the severity of their illness and their baseline Hb: aRBCx would be preferred in case of severe illness, and ST would be indicated in case of Hb of <7 g/dL. Most panelists (11 of 12, 91.7%) would recommend continuing prophylactic transfusions throughout the pregnancy after an episode of ACS. However, there was no alignment on the recommended interval between transfusions (Table 3): over half of the panelists (7 of 12, 58.3%) responded that they would decide on a case-by-case basis.
Most panelists (10 of 12, 83.3%) would recommend a transfusion in the acute setting for life-threatening events, even in the presence of patient- or disease-specific factors that would make them recommend against prophylactic transfusions in the outpatient setting (eg, a history of serious transfusion reactions, or multiple alloantibodies).
Panelists agreed that intrauterine growth restriction (11 of 12, 91.7%) and fetal distress because of maternal complications (in the absence of an indication for delivery; 9 of 12, 75.0%) represented complications that would prompt them to recommend transfusion in the acute setting, but their decision-making process would factor in the risk-to-benefit ratio between the mother and fetus. Consensus was not reached for uteroplacental failure or oligohydramnios as indications for transfusion (Table 3). Two panelists would defer this decision to OB/GYN specialists.
Panelists strongly agreed (11 of 12, 91.7%) that, if feasible from a pain management standpoint, patients under chronic opioid treatment should limit or taper opioids from conception until completion of breastfeeding, to reduce the risk of neonatal abstinence syndrome and harmful effects on the newborn. Moreover, all panelists would recommend starting such patients on prophylactic RBC exchange to reduce the frequency of acute pain episodes, while limiting their opioid exposure. However, panelists mentioned that opioids should not be inadvertently withheld from patients who continue to experience pain while receiving prophylactic transfusions.
Delivery and follow-up
Consensus was not reached regarding the Hb level that should be targeted before delivery, with 5 of 12 experts (41.7%; Table 4) indicating that this should be determined on a case-by-case basis. However, most panelists (9 of 12, 75.0%) agreed that the targeted level would not differ between vaginal delivery or cesarean section, assuming all other variables are the same.
Most panelists (8 of 12, 66.7%) would prefer ST over other transfusion modalities (ie, mRBCx [modified/partial] or aRBCx) for a pregnant individual with SCD not receiving prophylactic transfusion therapy during pregnancy but who needed transfusion before delivery; however, consensus was not reached. Panelists who neither agreed nor disagreed with this statement indicated their decision would depend on the Hb level.
More than half of the experts (7 of 12, 58.3%) agreed that in the absence of maternal and/or fetal complications, spontaneous labor should be awaited up to 40 weeks of gestation, and half disagreed with inducing labor or planning a cesarean section at 36 to 38 weeks of gestation; the results were consistent across specializations. However, a consensus was not reached (Table 4). Panelists strongly agreed (11 of 12, 91.7%) that the decision for labor induction or elective cesarean section should be taken by a multidisciplinary team including OB/GYN specialists, hematologists, neonatologists, anesthesiologists, and blood bank specialists.
Most panelists (10 of 12, 83.3%) also agreed that in addition to the standard obstetric indications, there are no SCD-specific indications for electing cesarean section over vaginal delivery for pregnant patients with SCD.
Bottlenecks and knowledge gaps
There was no consensus regarding the top priority for research on the use of prophylactic transfusions in the management of pregnant individuals with SCD, but half of the panelists indicated they would prioritize research focused on the risk-to-benefit ratio of routine prophylactic transfusion therapy in all pregnant individuals with SCD. Research topics regarding the optimal modality for prophylactic transfusion and the best timing for starting prophylactic transfusion during pregnancy would each be prioritized by 3 panelists.
Discussion
Although standardized approaches exist for several aspects of SCD management in pregnancy, only few recommendations can be formulated based on strong evidence, because of lack of data. In these areas, practicing clinicians need to rely on their best clinical judgment, leading to differences in the healthcare services received by pregnant people with SCD. Through this international, multidisciplinary Delphi panel, we attempted to elaborate recommendations based on consensus for those topics for which published evidence to guide clinical practice is scarce.
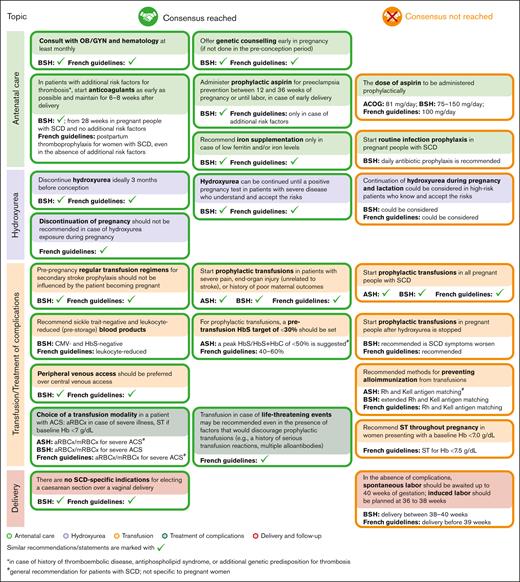
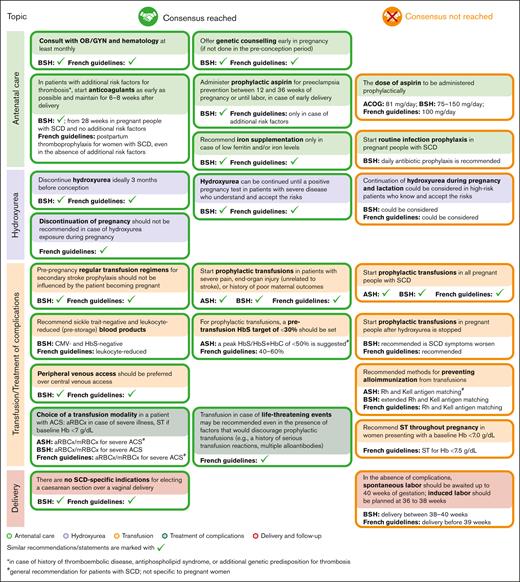
In several areas of SCD management, consensus was achieved, and recommendations for healthcare providers were formulated (Figure 2). The alignment between the areas of consensus of this Delphi panel and the recommendations of several professional societies/national guidelines relevant for SCD management is presented in Figure 3.
Alignment between selected areas of consensus and nonconsensus of the Delphi survey and the recommendations of professional societies/national guidelines. Recommendations of professional societies and national guidelines were retrieved from ACOG,16 ASH,15 BSH,7 and the French guidelines.14 ACOG, the American College of Obstetricians and Gynecologists; ASH, the American Society of Hematology; CMV, cytomegalovirus.
Alignment between selected areas of consensus and nonconsensus of the Delphi survey and the recommendations of professional societies/national guidelines. Recommendations of professional societies and national guidelines were retrieved from ACOG,16 ASH,15 BSH,7 and the French guidelines.14 ACOG, the American College of Obstetricians and Gynecologists; ASH, the American Society of Hematology; CMV, cytomegalovirus.
In general, the areas of nonconsensus seemed to correspond to areas with the widest knowledge gaps and lack of evidence. One such area is represented by the question of whether continuation of hydroxyurea therapy could be considered during pregnancy and lactation in patients with severe disease who are informed of the potential risks. In a recent study, hydroxyurea use for pregnant individuals with SCD was associated with higher odds of miscarriage, stillbirth, and low birthweight but not with serious medical problems at birth10; nevertheless, no increased frequency of miscarriage or stillbirth after hydroxyurea exposure during pregnancy was observed in another study.25 Very limited observational data on hydroxyurea use during pregnancy showed no overt teratogenicity in humans25-28; however, to our knowledge, to date, no studies designed specifically to evaluate birth defect rates after hydroxyurea use by pregnant individuals with SCD have been published. The possibility of individuals with severe disease continuing hydroxyurea during pregnancy, provided that they discuss the risks and benefits with a multidisciplinary team, is acknowledged by the British Society for Haematology (BSH) guideline and the French guideline.7,14 In the future, results of the HELPFUL trial (www.clinicaltrials.gov identifier: #NCT04093986), which will analyze retrospective data on hydroxyurea exposure during pregnancy, could provide additional evidence to orient recommendations related to this topic.
Although panelists agreed on starting prophylactic aspirin therapy to lower the risk of preeclampsia if no contraindications were identified, consensus was not reached regarding the dose of aspirin to be administered prophylactically. Professional societies and national guidelines are not aligned either on this topic: the BSH guideline recommends a dose of 75-150 mg per day,7 the American College of Obstetricians and Gynecologists recommends a dose of 81 mg per day,16 and the French guidelines recommend a dose of 100 mg per day.14 In a study conducted in a non-SCD population, aspirin use during pregnancy was associated with increased (but still low) incidence of postpartum bleeding (10.2% vs 7.8%; adjusted odds ratio: 1.23; 95% confidence interval: 1.08-1.39), and potentially with neonatal intracranial hemorrhage (0.07% vs 0.01%; adjusted odds ratio: 9.66; 95% confidence interval: 1.88-49.48).29 However, for pregnant individuals with SCD, the risk-to-benefit ratio of prophylactic aspirin might be different from that in the non-SCD population. Results of the PIPSICKLE trial (www.clinicaltrials.gov identifier: #NCT05253781) could help to better define the benefits of low-dose aspirin administered to pregnant individuals with SCD.30
Consensus to support standard prophylactic transfusion therapy for all pregnant people with SCD was not reached; this is consistent with the American Society of Hematology15 and BSH7 recommendations. However, experts did identify several scenarios in which prophylactic transfusion therapy should be initiated, both to prevent and to treat SCD complications, especially acute pain episodes. This recommendation reflects the limited but encouraging data regarding the benefits of prophylactic transfusions for pregnant individuals with SCD: results of a randomized controlled trial and of a meta-analysis both showed a reduction of recurrent acute vaso-occlusive pain episodes in pregnant patients with SCD who received prophylactic transfusions.13,31 The results of the TAPS2 trial (www.clinicaltrials.gov identifier: #NCT03975894) investigating this topic, should provide more evidence of any potential benefit from prophylactic transfusions during pregnancy.32,33
Within the topic of prophylactic transfusions, consensus was not achieved regarding the frequency of prophylactic transfusions, target hematocrit levels, or the recommended methods for preventing alloimmunization, although all panelists would recommend at least 1 method. Results of a pediatric study suggest that using aRBCx instead of ST could also lower the alloimmunization rate in children with SCD.34
The numerous areas of nonconsensus related to prophylactic transfusions in pregnant individuals with SCD highlight the need for further research in this area. The experts also highlighted additional research topics of interest among those not addressed in this Delphi survey, such as the impact of maternal anemia, alloimmunization, and opioid use on neonatal outcomes or the benefit-to-risk ratio of steroid use for fetal lung maturation for individuals with SCD.
Although the Delphi process has increasingly been used to optimize medical decision making in several areas of healthcare, including inherited blood disorders,35 to our knowledge, this is the first study to apply this technique for obtaining consensus in a comprehensive range of topics pertaining specifically to the management of SCD for pregnant people. Although the number of panelists who participated in the survey is limited, they represent a multidisciplinary team of experts from several upper middle– and high-income countries, ensuring that their responses reflect best clinical practice rather than local standard of care. A notable limitation of the study is that experts from regions/countries with high SCD incidence (such as sub-Saharan Africa or India) were not involved. Similar studies conducted in the future could aim to include specialists with a more diverse geographical background, and to elaborate differentiated recommendations for each pregnancy trimester.
The recommendations formulated here could be used as a guide for healthcare professionals treating pregnant individuals with SCD, although these guidelines might need to be adapted to the resources available in each setting. In addition, the knowledge gaps identified can inform the development and design of much needed clinical trials in the field.
Acknowledgments
The authors thank the Akkodis Belgium platform for assistance during the Delphi process, project coordination, and editorial and design support, on behalf of Terumo Blood and Cell Technologies. Timea Kiss provided medical writing support and Sophie Timmery provided project coordination. Lotte Mathé, Petronela M. Petrar, and Alpár Pöllnitz were involved in developing the Delphi surveys and summarizing the results. The authors also thank Mickael Beraud (Terumo Blood and Cell Technologies) for project oversight. The authors thank France Pirenne for her valuable contribution during the first round of the Delphi survey.
This work was supported by Terumo Blood and Cell Technologies. Terumo Blood and Cell Technologies provided funding for conducting the study and for manuscript development but was not involved in creating the surveys or in interpreting the answers of the experts.
Authorship
Contribution: D.S., I.K., and J.H. developed the survey questions; and all authors contributed to data collection (by answering the items of the 2 Delphi surveys) and data interpretation, and critically revised the manuscript.
Conflict-of-interest disclosure: D.S., I.K., and J.H. received consulting fees from Terumo Blood and Cell Technologies for their participation in the survey creation and interpretation. Outside of the submitted work, J.H. has taken up employment with Vertex Pharmaceuticals subsequent to the work carried out in this article. S.L. is the vice president of the National Alliance of Sickle Cell Centers. S.P. received personal fees for lectures from Novartis and from Celgene for serving on an advisory board; and has received support from Celgene and Vertex Pharmaceuticals for attending meetings. L.H.P. received grants from the National Institutes of Health and the National Heart, Lung, and Blood Institute (K23HL146841 and U01HL156620-01), the Doris Duke Charitable Foundation (grant #2020147), the Mellon Foundation, Alexion, and the American Society of Hematology; has received consulting fees from Global Blood Therapeutics and Novo Nordisk; received support from the American Society of Hematology and the Hemostasis and Thrombosis Research Society for attending meetings; reports participation in a data safety monitoring board (CRESCENT [CRISPR SCD001]); and is the cofounder and vice chair of the Medical Advisory Board of the Sickle Cell Reproductive Health Education Directive, a member of the Maternal Health Working Group (American Society of Hematology), and cochair of the PhenX Genitourinary Working Group. The remaining authors declare no competing financial interests.
The current affiliation for S.Y.B. is the Department of Obstetrics and Gynecology, Dr. Sinasi Can Hospital, Acibadem Healthcare Group, Istanbul, Turkey.
The current affiliation for J.H. is Vertex Pharmaceuticals Incorporated, Boston, MA.
Correspondence: Deva Sharma, Vanderbilt University Medical Center, 1211 Medical Center Dr, Nashville, TN 37232; email: deva.sharma@vumc.org.
References
Author notes
D.S. and I.K. contributed equally to this study.
Presented, in part, at the 64th annual meeting of the American Society of Hematology, New Orleans, LA, 10-13 December 2022.
Data are available on request from the corresponding author, Deva Sharma (deva.sharma@vumc.org).
The full-text version of this article contains a data supplement.