In this issue of Blood Advances, Mehta et al1 described a study aimed at dissecting the relative importance of donor age and HLA-DP matching status in the outcome of matched unrelated donor hematopoietic cell transplantation (HCT). Both factors are well known for their impact on the success of allogeneic HCT. Consequently, they are included in the national and international guidelines for donor selection, along with other variables such as cytomegalovirus serostatus, donor/recipient sex matching, ABO compatibility, and more.
The numerous variables affecting clinical outcomes, either increasing toxicity or improving protection from disease reoccurrence, present a challenge in understanding their interactions and the establishment of priorities to guide the selection of the best donor available. The study by Mehta et al contributes to filling this gap by providing evidence supporting the superior impact of donor age on clinical outcomes, and its modulation by different degrees of HLA matching, particularly when a young donor is selected. This study reinforces with evidence the current donor selection recommendations.
The authors leveraged a data set previously published by the Center for International Blood and Marrow Transplant Research, which included a cohort of 10 783 patients receiving HCT from 10 of 10 HLA-matched unrelated donors as a treatment for acute myeloid or lymphoblastic leukemia or myelodysplastic neoplasm between 2008 and 2018.2 This cohort was split into 6 groups based on donor age and patient/donor HLA-DP matching status; the first group was dichotomized into young and old donor categories by the cutoff age of 35 years, and the second group was analyzed using the T-cell epitope matching model3 to discriminate between HLA-DP allele matched or permissive and nonpermissive mismatched donors. For the main association analysis, the group of patients who underwent transplantation with HLA-DP matched/young donors was used as the reference group.
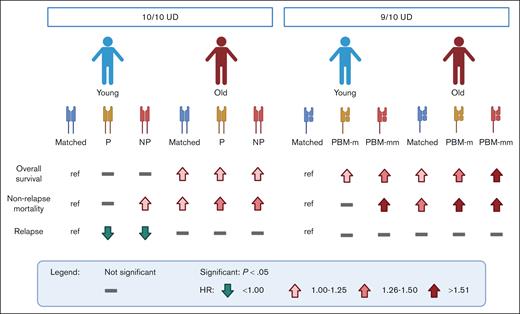
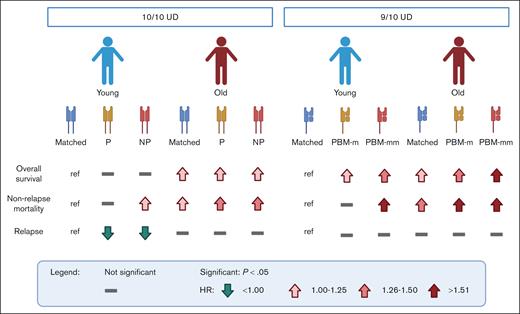
The granular analysis performed by Mehta et al revealed that patients who underwent transplantation with older donors generally have a higher risk of mortality (see figure), with both permissive and nonpermissive HLA-DP mismatched groups experiencing increased incidences of acute and chronic graft-versus-host disease (GVHD). Based on this, older donors should be avoided regardless of their HLA-DP matching status; even a young donor with a nonpermissive HLA-DP mismatch might be preferable to any older donor. Within the group of patients who underwent transplantation with young donors, HLA-DP matching status modulated the risk, similar to that in previous reports.4 Permissive mismatched donors were preferable compared with others, as they resulted in a slightly better but not significant chance of survival due to reduced relapse incidence compared with HLA-DP matched donors, without concomitant increase in the risk of nonrelapse mortality and GVHD seen in patients who underwent transplantation with nonpermissive mismatched donors.
Association of HLA matching and donor age with clinical outcomes of allogeneic HCT from unrelated donors (UDs). Schematic representation of the risk associated with different combinations of donor age (young, ≤35 years; old, >35 years) and HLA-DP matching status by T-cell epitope in 10 of 10 HLA-matched UDs1 or HLA class I matching status by PBM in 9 of 10 HLA-matched UDs.8 Lines and arrows indicate not significant difference compared with the reference (ref), or significantly (P < .05) higher (in red) or lower (in green) hazard ratio (HR) as depicted in the symbol legend. Matched, allele matched; NP, HLA-DP nonpermissive mismatched; P, HLA-DP permissive mismatched; PBM-m, HLA class I PBM matched; PBM-mm, HLA class I PBM mismatched. Figure created with BioRender.com.
Association of HLA matching and donor age with clinical outcomes of allogeneic HCT from unrelated donors (UDs). Schematic representation of the risk associated with different combinations of donor age (young, ≤35 years; old, >35 years) and HLA-DP matching status by T-cell epitope in 10 of 10 HLA-matched UDs1 or HLA class I matching status by PBM in 9 of 10 HLA-matched UDs.8 Lines and arrows indicate not significant difference compared with the reference (ref), or significantly (P < .05) higher (in red) or lower (in green) hazard ratio (HR) as depicted in the symbol legend. Matched, allele matched; NP, HLA-DP nonpermissive mismatched; P, HLA-DP permissive mismatched; PBM-m, HLA class I PBM matched; PBM-mm, HLA class I PBM mismatched. Figure created with BioRender.com.
The increased risk of mortality associated with HCT in older HLA-matched unrelated donors might be explained by a reduction in immune function due to aging.5 The aged immune system of the donor might be less efficient in responding to pathogens and less educated by the thymus, leading to reduced depletion of potentially alloreactive T cells. Overall, the increased risk associated with older donor age appeared to mask the differential impact of HLA-DP match, permissive and nonpermissive mismatches.
Stratification of HLA-DP mismatches is based on the divergence of peptide repertoires (immunopeptidomes) presented by patient and donor allotypes.6 Permissive mismatches are characterized by low immunopeptidome divergence, thus presenting only a limited number of allopeptides that are not shared between patients and donors and are potential targets of alloreactivity. The opposite is expected for the highly divergent HLA-DP allotypes involved in nonpermissive mismatches. The understanding of this molecular mechanism at the basis of HLA-DP permissiveness led to a refinement of the previous matching algorithm, enabling discrimination between permissive “core” and “noncore” mismatches defined by low and high immunopeptidome divergence, respectively, and associated with different clinical outcomes.7 In their study, Mehta et al did not use this new updated algorithm, which might provide better accuracy in the analysis of the interaction between donor age and HLA-DP matching. It will be interesting to see future studies challenging the relationship between donor age and this new approach to HLA-DP matching, as well as investigating other HLA-matching models.
An example in this direction was already provided by the same authors in a previous issue of Blood Advances.8 A similar model based on the concept of immunopeptidome divergence was recently introduced for grading HLA class I mismatches, in which peptide-binding motifs (PBMs) were used to predict immunopeptidome divergence.2 Mismatches involving alleles with different PBMs (PBM mismatched) are less tolerated than those with similar PBMs (PBM matched). Mehta et al investigated the interaction between donor age and PBM matching status showing that these 2 variables have a combined effect.8 Young donors again represented the most preferable option; however, within both young and old donors, PBM mismatches were associated with poorer clinical outcomes compared with PBM matches (see figure).
Mehta et al provided important new insights into the relationship between HLA-matching models (both for HLA-DP1 and HLA class I8) and donor age. Nevertheless, both studies had a major limitation in that they investigated this important question in patients treated with calcineurin-based GVHD prophylaxis. Although still common in clinical practice, this approach has been progressively replaced by the use of posttransplant cyclophosphamide (PTCy) as the new standard for GVHD prophylaxis. PTCy reduces the HLA barrier of allogeneic HCT, making transplantation from HLA-mismatched unrelated donors a feasible option, therefore expanding the pool of available donors. In this setting, the impact of HLA matching is currently under debate,9,10 and future investigations will help clarify its role, particularly the possibility that a minimum threshold of immunogenicity from HLA mismatches might be required to guarantee protection from malignant disease relapse. The results of Mehta et al in this context contributed to shed new light on the complexity of factors determining the clinical outcome of allogeneic HCT. Certainly, this and future investigations, also taking into account different regimens of GVHD prophylaxis, heterogeneity of the disease, disease risk, and other factors, will help better define the characteristics to look for during the selection of the best “good Samaritan” for each individual patient.
Conflict-of-interest disclosure: P.C. declares no competing financial interests.