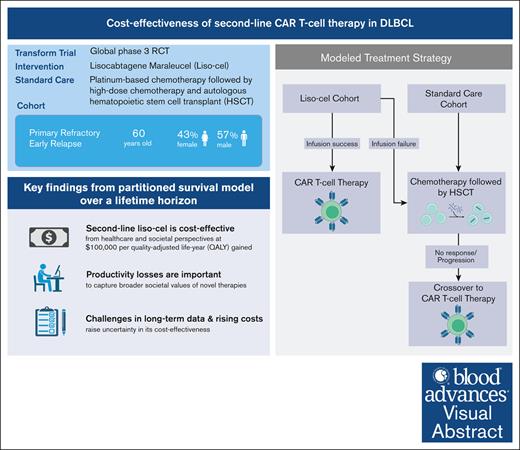
Liso-cel shows cost-effectiveness in second-line DLBCL therapy with health care and societal ICERs at $99 669 and $68 212, respectively.
Study shows liso-cel benefits, but rising costs and scarce long-term data challenge its cost-effectiveness at $100 000 per QALY.
Visual Abstract
Lisocabtagene maraleucel (liso-cel), a chimeric antigen receptor (CAR) T-cell therapy, received the US Food and Drug Administration approval in 2022 for second-line treatment of diffuse large B-cell lymphoma (DLBCL) for patients with refractory disease or early relapse after first-line chemoimmunotherapy. This decision was based on the TRANSFORM study demonstrating improvements in event-free survival with liso-cel compared with standard care. Given the high costs of CAR T-cell therapies, particularly as they transition to second-line treatment, a cost-effectiveness analysis is essential to determine their economic viability. The study used a partitioned survival model with standard parametric functions to evaluate the cost-effectiveness of liso-cel aganist platinum-based chemotherapy followed by high-dose chemotherapy and autologous hematopoietic stem cell transplantation over a lifetime horizon The analysis relied on data from the TRANSFORM and TRANSCEND trials, established literature, and public data sets to calculate the incremental cost-effectiveness ratio (ICER). For a representative cohort of US adults aged 60 years, ICER of liso-cel was $99 669 per quality-adjusted life-year (QALY) from a health care sector perspective and $68 212 per QALY from a societal perspective, confirming its cost-effectiveness at the $100 000 per QALY threshold. Nonetheless, under certain scenarios, liso-cel surpasses this benchmark but remains within the US acceptable range of $150 000 per QALY. A key finding underlines the importance of incorporating productivity losses into such analyses to capture the broader societal values of novel therapies. Although these therapies offer substantial clinical benefits, their high acquisition costs and limited long-term data critically challenge their economic sustainability.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma.1 Although patients with DLBCL may achieve long-term remission when treated with first-line chemoimmunotherapy, ∼30% to 50% of patients with DLBCL experience relapsed or refractory (R/R) disease.2,3 The conventional treatment for R/R DLBCL involves platinum-based chemotherapy, followed by high-dose chemotherapy and autologous hematopoietic stem cell transplantation (HSCT) for eligible patients who are chemosensitive.4 However, this strategy has only cured a small subset of patients with R/R DLBCL, and those who experience early relapse or are primarily refractory to treatment have significantly worse outcomes.5
In 2022, 2 chimeric antigen receptor (CAR) T-cell therapies, axicabtagene ciloleucel (axi-cel) and lisocabtagene maraleucel (liso-cel), received approval for use as second-line treatments for patients with DLBCL who either exhibited primary refractory disease or experienced relapse within 12 months after initial chemoimmunotherapy. The approval of axi-cel and liso-cel as second-line therapies was based on their demonstrated efficacy in the ZUMA-7 and TRANSFORM studies, respectively. In the ZUMA-7 study, axi-cel achieved a median event-free survival (EFS) of 10.8 months compared with 2.3 months for standard care (SC), and a median progression-free survival (PFS) of 14.7 months compared with 3.7 months for SC.6 Over a median follow-up of 47.2 months, axi-cel attained a complete response (CR) rate of 65%, compared with 32% for SC. Moreover, axi-cel showed notable improvement in overall survival (OS), with a 4-year estimated OS of 54.6%, compared with 46% for the SC group. Similarly, in the TRANSFORM study, liso-cel showed superior efficacy over SC, with a median follow-up of 17.5 months. For liso-cel, the median for both EFS and PFS was not reached, in contrast to 2.4 months (EFS) and 6.2 months (PFS) for SC. Furthermore, liso-cel achieved a 74% CR rate compared with 43% with SC, and also demonstrated a neumerical improvement in 18-month OS of 73% for liso-cel compared with 54% for SC.7
The transition of CAR T-cell therapies such as axi-cel and liso-cel to earlier lines of therapy brings both potential benefits and economic challenges. Although these therapies offer considerable clinical advantages, their acquisition costs are rising notably; axi-cel increased from $399 000 in 2022 to $424 000 in 2023 and liso-cel from $410 300 to $447 227 over the same period.8 Beyond the acquisition costs, the overall financial impact is compounded by the total health care expenditure, which includes hospitalization costs, posttreatment care, and management of side effects. These escalating costs can create barriers to access, particularly for patients from lower socioeconomic backgrounds or those without comprehensive insurance coverage.9 The financial burden associated with these therapies could impede their integration into clinical practice, hindering access to potentially life-saving treatments, and diminishing their societal benefits.10 Therefore, it is crucial to continually evaluate their cost-effectiveness and overall value,11 taking into account the current uncertainties in evidence12 and their potential long-term impact on patient outcomes.
Axi-cel has already demonstrated cost-effectiveness as a second-line treatment, meeting the $100 000 per quality-adjusted life-year (QALY) threshold.13-15 Although both axi-cel and liso-cel are approved for the same indication, liso-cel carries a higher list price and has shown an increased incidence of severe adverse events in the TRANSFORM study, compared with axi-cel in the ZUMA-7 study. Furthermore, as CAR T-cell therapies transition to second-line treatment, they are likely to expand the eligible patient population, thereby affecting health care budgets. As such, formal assessment of the cost-effectiveness of liso-cel is essential. Importantly, our analysis bridges a critical gap in existing research by including productivity loss data, offering a more comprehensive societal view on the value of CAR T-cell therapies. This study aims to evaluate the cost-effectiveness of liso-cel as a second-line treatment compared with SC chemotherapy and autologous HSCT, from both health care sector and societal perspectives in the United States.
Methods
This economic evaluation is exempt from review and informed consent by the University of Southern California, because it does not involve human participant research. It adheres to the Consolidated Health Economic Evaluation Reporting Standards16 and the US Second Panel on Cost-effectiveness in Health and Medicine recommendations.17
Treatment strategies
We constructed a cost-effectiveness model to compare liso-cel with the SC regimen as a second-line therapy for DLBCL. The SC regimen primarily consists of platinum-based chemotherapy, followed by high-dose chemotherapy and autologous HSCT.
The primary outcomes of our study were life-years gained and QALYs. These were derived from the long-term survival data of the TRANSFORM and TRANSCEND clinical trials. TRANSFORM is a global phase 3 study that examined the efficacy of liso-cel as a second-line treatment for patients with primary refractory or early relapsed (≤12 months) LBCL.7,18 The TRANSCEND trial assessed liso-cel as a third-line therapy for patients with LBCL with more complex clinical profiles, including moderate comorbidities and secondary central nervous system involvement.19
Our model used a hypothetical cohort of patients with DLBCL, aged 60 years with 57% male representation, consistent with the demographic profile in the TRANSFORM study (supplemental Methods 1). This group consisted of patients with refractory DLBCL or those who relapsed early, within 12 months of initial treatment. Such an approach was chosen due to the lack of access to individual patient data, necessitating reliance on aggregated EFS and OS data for analysis.
Treatment regimens mirrored those in the TRANSFORM trial (supplemental Methods 2). The liso-cel group underwent lymphodepleting chemotherapy using IV fludarabine and cyclophosphamide, followed by liso-cel administration. Our model considered a 3% failure rate in CAR T-cell infusion, as observed in TRANSFORM, leading to a shift to SC for these patients. Furthermore, 63% of the liso-cel group received an optional bridging therapy cycle. For the SC arm, the model allowed for transitioning to third-line CAR T-cell therapy if initial treatments failed, a scenario that applied to 52% of these patients.
Model structure and calibration
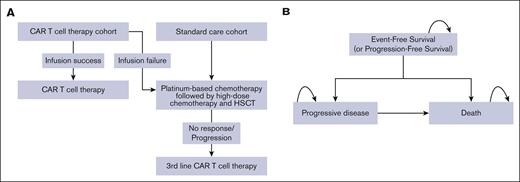
We developed a partitioned survival model to represent the patient pathways after treatment with 3 distinct states (Figure 1A-B). The first group, based on the TRANSFORM study, included states of EFS, progressive disease (PD), and death. The crossover cohort, transitioning to liso-cel as a third-line treatment, had states of PFS, PD, and death, with PFS data derived from the TRANSCEND study.
Model structure. (A) Treatment strategies for the liso-cel group and the SC group. (B) Partitioned survival model. Once patients entered a partitioned survival model’s state, they either remained in the same state (self-arrow) or progressed to later states.
Model structure. (A) Treatment strategies for the liso-cel group and the SC group. (B) Partitioned survival model. Once patients entered a partitioned survival model’s state, they either remained in the same state (self-arrow) or progressed to later states.
A standard parametric survival function was used to extrapolate survival data from both TRANSFORM and TRANSCEND studies. The selection of the most appropriate models for long-term EFS, PFS, and OS was guided by the lowest Akaike information criterion and Bayesian information criterion values. Model calibration involved aligning these functions with empirical survival data from the 2 main studies. Transition probabilities were then extracted from these calibrated curves. For patients maintaining EFS or PFS for >40 months, a phase indicative of a survival plateau with other CAR T-cell therapies,20,21 the occurrence of relapse was perceived as highly unlikely. This aligns with observed long-term outcomes in patients with DLBCL treated with immunochemotherapy, based on 60-month median follow-up data.22
The incremental cost-effectiveness ratio (ICER), calculated as the cost per incremental QALY gained, compared liso-cel with SC at the willingness-to-pay threshold of $100 000 per QALY. Our study adopted a lifetime horizon to account for the curative potential of CAR T-cell therapies. All costs were adjusted to 2022 US dollars (USD), and a 3% annual discount rate was applied to both costs and QALYs.17
Model parameters
Input parameters for our economic model were sourced from the key liso-cel trials, peer-reviewed literature, and public databases18,22-44 (Table 1).
Direct medical costs from a health care sector perspective included expenses related to routine CAR T-cell procedures, chemotherapy regimens, autologous HSCT, inpatient and intensive care unit admissions, postinfusion monitoring, and management of disease progression and end-of-life care. These costs were based on literature relevant to our target cohort and supplemented with data from the Centers for Medicare and Medicaid Services. Costs of managing serious adverse events (grade ≥3), occurring in ≥5% of TRANSFORM study participants, were included in the analysis. The model also considered costs for managing B-cell aplasia, experienced by 16% of liso-cel recipients in the TRANSFORM study.
From a societal perspective, indirect costs such as lost productivity due to mortality and direct out-of-pocket expenses related to travel were included. Productivity losses were segmented into 3 categories: lost labor market earnings, unpaid productivity loss, and the cost of uncompensated household activities, following the recommendation of the US Second Panel on Cost-effectiveness in Health and Medicine17 (supplemental Methods 3; supplemental Table 1). The lost labor market earnings were calculated using the human capital approach, drawing upon multiple metrics from the US Bureau of Labor Statistics.31,34,35,37 Unpaid productivity loss was estimated using participation rates and weekly hours dedicated to volunteering and caregiving for nonhousehold members from the Health and Retirement Study (HRS),31,32,36 applying median age–specific wages to individuals aged >70 years.32 The cost of uncompensated household activities was assessed by valuing time spent on babysitting grandchildren, as reported in the HRS.31,32,36 We assumed a retirement age of 67, aligning with the age for full social security benefits. Moreover, our analysis factored in the travel-related expenses and time commitments of patients and caregivers for both SC and CAR T-cell therapy, with the latter's costs sourced from academic institutions and community multispecialty hospitals.29
For the quality-of-life assessments, we included utility scores of 0.85 for patients in the EFS state and 0.78 for those in the PD state, derived from EuroQol-5 Dimensions (EQ-5D) data in the TRANSCEND study.33 Furthermore, we incorporated disutility values to reflect temporary quality-of-life declines during treatments: −0.15 for 1 month of CAR T-cell therapy, −0.15 for 3 months of platinum-based chemotherapy, and −0.15 for 1 month after autologous HSCT.38
Sensitivity analyses
The study used a univariate sensitivity analysis, adjusting each parameter by ±25%, to assess their individual impact on cost-effectiveness. Additionally, a Bayesian multivariate probabilistic sensitivity analysis with 10 000 Monte Carlo simulations was conducted to evaluate uncertainty across all parameters, accounting for their inherent random variations.
Scenario and threshold analyses
First, we implemented a 5-year time horizon analysis reflecting the present extent of clinical data and compared these findings with the lifetime horizon, providing perspective on short-term vs long-term cost-effectiveness. Second, we evaluated the impact of alternative utility values for patients with R/R DLBCL using 0.83 for those in EFS/PFS and 0.39 for those in PD states on the overall results.45 Third, the model assumption was extended from transitioning patients to the long-term DLBCL survival curve after 40 months in EFS/PFS to a 5-year period, assessing the model's robustness in longer-term scenarios. Fourth, to quantify the potential impact of temporal price fluctuations on the ICER, we applied the 2023 list price of liso-cel while maintaining all other cost parameters in 2022 USD. Finally, a threshold analysis identified cost-effective price points for CAR T-cell therapy, based on a willingness-to-pay threshold of $100 000 per QALY. This analysis was essential for evaluating the economic viability of CAR T-cell therapy within set budget constraints.
Results
Survival outcomes
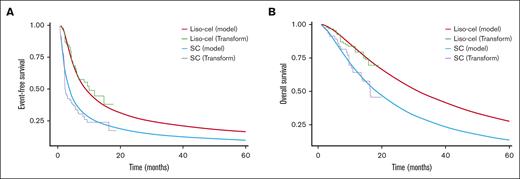
Using a standard parametric survival function, our model revealed distinct differences in survival outcomes between liso-cel and SC. The EFS rate at 40 months after treatment was estimated at 23% for liso-cel recipients, in contrast to a 10% EFS rate for those receiving SC (Figure 2A). In terms of OS, the rates at the same 40-month interval stood at 45% for the liso-cel cohort and 22% for SC patients (Figure 2B). Extending the evaluation to a 5-year period, the EFS rate for liso-cel was 18%, compared with 8% for SC. Furthermore, the 5-year OS rate was calculated at 32% for patients treated with liso-cel, whereas it was 12% for those receiving SC.
Survival analysis. (A) Modeled EFS using a parametric survival function. (B) Modeled OS using a parametric survival function.
Survival analysis. (A) Modeled EFS using a parametric survival function. (B) Modeled OS using a parametric survival function.
Base case analysis
From a US health care sector perspective, the analysis revealed that the total discounted lifetime health care costs for liso-cel amounted to $668 624, in contrast to $467 624 for SC (Table 2). This evaluation considers the overall financial burden on the health care system over the patients' lifetimes, factoring in the costs associated with the respective treatment modalities.
From a US societal perspective, the analysis encompasses a broader range of societal expenses. Discounted travel-associated costs for patients and their caregivers were calculated at $10 458 for liso-cel and $14 425 for SC. Additionally, the impact of mortality on labor market earnings was assessed, with lost earnings due to mortality amounting to $110 608 for liso-cel and $156 362 for SC. Unpaid productivity loss was estimated at $34 347 for liso-cel and $39 426 for SC. Furthermore, uncompensated household production costs were calculated at $58 437 for liso-cel and $67 078 for SC.
In terms of quality of life and life expectancy analysis, patients receiving liso-cel therapy exhibited an average life expectancy of 5.34 years, compared with 2.47 years for those on SC. Additionally, patients receiving liso-cel achieved an average of 3.64 QALYs compared with 1.62 QALYs for SC recipients (Table 3).
The ICER for liso-cel was $99 669 per QALY from a health care sector perspective and $68 212 per QALY from a societal perspective. These values position liso-cel as a cost-effective option at the widely accepted threshold of $100 000 per QALY from both perspectives.
Sensitivity analyses
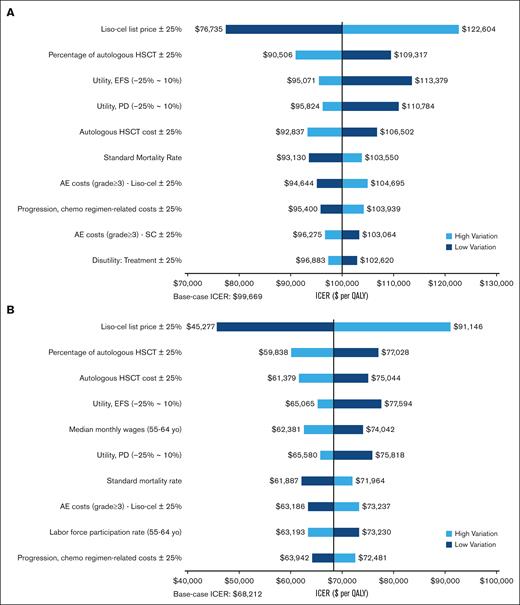
The univariate sensitivity analyses revealed that the cost-effectiveness of liso-cel, evaluated at a $100 000 per QALY threshold from a health care sector perspective, was influenced by various factors. These included the list price of liso-cel, the rates and costs associated with autologous HSCT, utility values for EFS and PD states, and the costs related to managing adverse events (Figure 3A). From a societal perspective, the cost-effectiveness of liso-cel at the $100 000 per QALY threshold was robust against parameter uncertainties (Figure 3B).
Univariate sensitivity analyses. (A) Health care sector perspective. (B) Societal perspective. AE, adverse event; Chemo: Chemotherapy.
Univariate sensitivity analyses. (A) Health care sector perspective. (B) Societal perspective. AE, adverse event; Chemo: Chemotherapy.
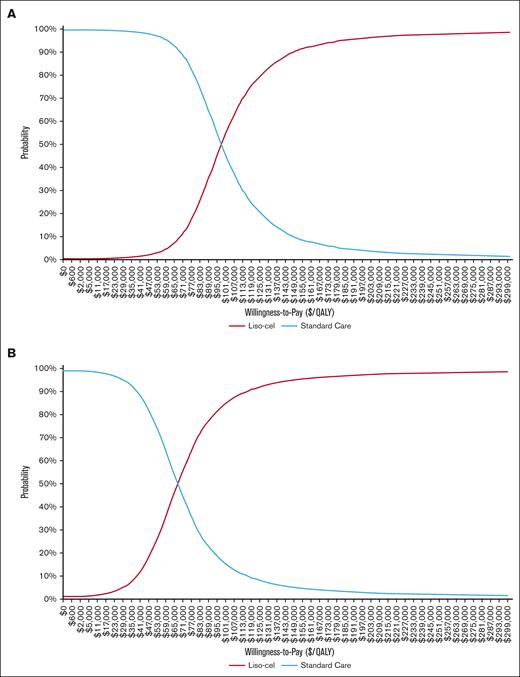
The multivariate probabilistic sensitivity analysis, conducted with 10 000 Monte Carlo simulations, assessed the cost-effectiveness of second-line liso-cel from a health care sector perspective at different willingness-to-pay thresholds. The results indicated a 3% probability of cost-effectiveness at $50 000 per QALY, increasing to 54% at $100 000 per QALY, and 90% at $150 000 per QALY. From a societal perspective, the probabilities of cost-effectiveness at these thresholds were 22%, 84%, and 95%, respectively (Figure 4).
Cost-effectiveness acceptability curves based on various willingness-to-pay thresholds. This figure depicts cost-effectiveness acceptability curves for liso-cel, which represent the likelihood of liso-cel being a cost-effective treatment at various willingness-to-pay thresholds. The curves were derived from a multivariate probabilistic sensitivity analysis over 10 000 Monte Carlo simulations. The curves are presented from 2 perspectives: (A) the health care sector and (B) the societal perspective.
Cost-effectiveness acceptability curves based on various willingness-to-pay thresholds. This figure depicts cost-effectiveness acceptability curves for liso-cel, which represent the likelihood of liso-cel being a cost-effective treatment at various willingness-to-pay thresholds. The curves were derived from a multivariate probabilistic sensitivity analysis over 10 000 Monte Carlo simulations. The curves are presented from 2 perspectives: (A) the health care sector and (B) the societal perspective.
Scenario analyses
In the scenario analysis with a 5-year horizon, the cost-effectiveness of second-line liso-cel varied between health care sector and societal perspectives. From a health care sector perspective, the analysis revealed an incremental cost of $133 249 and an incremental QALY of 0.97, resulting in an ICER of $136 757 per QALY (Table 4). In contrast, from a societal perspective, liso-cel demonstrated an incremental cost of $81 918 and an incremental QALY of 0.97, leading to an ICER of $84 075 per QALY (Table 4).
In the scenario analysis applying alternative utility values for R/R DLBCL, there was a decrease in incremental QALYs, dropping from 2.02 in the base case to 1.46 (Table 4). This change resulted in an increased ICER, rising to $137 856 per QALY from a health care sector perspective and to $94 346 per QALY from a societal perspective. Although second-line liso-cel remained cost-effective at the $100 000 per QALY willingness-to-pay threshold from a societal perspective, it achieved cost-effectiveness at a higher threshold of $150 000 per QALY from a health care sector perspective.
In the scenario extending DLBCL survival from 40 months to 5 years, the liso-cel group showed an incremental gain of 2.02 QALYs. Relative to the base case, this extension incurs an additional cost of $199 147, with a marginal QALY gain of just 0.02. The calculated ICERs were $98 661 per QALY from the health care sector perspective and $67 232 per QALY from the societal perspective (Table 4).
A scenario analysis using the 2023 list price of liso-cel, while keeping other costs in 2022 USD, yielded an ICER of $107 926 per QALY from a health care sector perspective and $76 468 per QALY from a societal perspective (Table 4).
Price threshold analysis
In this analysis, the highest price point at which liso-cel remains cost-effective was determined. From a health care sector perspective, liso-cel maintained its cost-effectiveness up to a price of $411 780 and from a societal perspective, up to $552 470. These figures are based on the $100 000 per QALY willingness-to-pay threshold and consider a lifetime horizon.
Discussion
This economic evaluation assessed the cost-effectiveness of liso-cel as a second-line therapy for patients with DLBCL experiencing refractory disease or early relapse within 12 months after first-line chemoimmunotherapy, compared with the conventional platinum-based chemotherapy followed by high-dose chemotherapy and autologous HSCT. Liso-cel showed improved survival outcomes over SC, as evidenced by higher EFS and OS rates at both 40-month and 5-year intervals. Furthermore, liso-cel patients experienced significantly higher QALYs than those receiving SC. The ICER values for liso-cel were $99 669 per QALY from a health care sector perspective and $68 212 per QALY from a societal perspective, indicating its cost-effectiveness against the $100 000 per QALY willingness-to-pay threshold from both perspectives over a lifetime horizon. A sensitivity analysis demonstrated that the cost-effectiveness of liso-cel is influenced by factors such as pricing and health care costs, with the upper ICER limit marginally exceeding the $100 000 per QALY threshold. Additionally, the probability of liso-cel being cost-effective increases at higher willingness-to-pay thresholds, notably from the societal perspective.
A series of scenario analyses were conducted in this study. One key scenario with a 5-year horizon revealed that liso-cel maintained cost-effectiveness from a societal perspective, with an ICER of $84 075 per QALY. However, from a health care sector perspective, the ICER increased to $136 757, surpassing the $100 000 threshold, suggesting that although liso-cel is not cost-effective at this level, it still remains within the acceptable range below the $150 000 per QALY threshold. Notably, the 5-year ICERs are not substantially different from those calculated over a lifetime horizon in our base case scenario. This suggests the potential for CAR T-cell therapies to maintain their value over time, regardless of the time frame used for the cost-effectiveness analysis. Additionally, extending the survival assumption to 5 years has only a minimal impact on the long-term cost-effectiveness of liso-cel. This is particularly significant given that the 40-month EFS and OS estimates used in this study are more conservative and lower than the 18-month data from the TRANSFORM study. The discrepancy likely arises from the use of standard parametric modeling, which may not fully capture long-term survival plateaus.46 Collectively, these scenario analyses provide valuable insights into the determinants affecting the cost-effectiveness of second-line liso-cel and highlight key areas for future research.
To the best of our knowledge, our study is the first to assess the cost-effectiveness of liso-cel as a second-line therapy for patients with DLBCL from health care sector and societal perspectives. A significant aspect of our analysis is the incorporation of productivity losses due to mortality, a factor frequently overlooked in previous cost-effectiveness studies of CAR T-cell therapies. Although our earlier research on axi-cel suggested that travel-related costs had a minimal impact on the ICER, primarily due to the high acquisition costs of the therapy,13 our current findings indicate a notable reduction in ICER for liso-cel when accounting for productivity losses. A further distinction of our study is the inclusion of unpaid activities such as volunteering, caregiving, and domestic responsibilities in our economic evaluation. Our approach tackles the age-related distributional bias common in analyses that only consider lost labor market earnings. It is especially relevant given the emphasis on an older cohort, whose significant yet often unquantified contributions to the informal labor market after retirement warrant recognition.47 By incorporating these broader economic factors, our analysis underscores the importance of evaluating productivity losses to more accurately assess the societal value of CAR T-cell therapies.
The US Food and Drug Administration clearance for second-line use of axi-cel and liso-cel prompts a critical examination of their value against high treatment costs. Previous studies, including our own, have established axi-cel's cost-effectiveness within the $100 000 per QALY threshold.13-15 However, the annual increase in the list price of axi-cel and liso-cel poses a challenge to their economic viability. Our sensitivity analysis suggests that liso-cel maintains cost-effectiveness with an acquisition price up to $411 780 from a health care sector perspective and $552 470 from a societal perspective. However, at its 2023 price point of $447 227, the ICER of liso-cel surpasses the $100 000 QALY threshold, registering at $107 926 per QALY from a health care sector perspective, but still within the broader US acceptability range up to $150 000 per QALY. An additional factor affecting the financial viability of CAR T-cell therapies is the mismatch between treatment costs and reimbursement rates. The 2023 Inpatient Prospective Payment System has established a maximum reimbursement of $356 134 for CAR T-cell therapies, a figure significantly below the list price of liso-cel, emphasizing the widening gap between treatments costs and health care system allocations.48 This could affect equitable access to these therapies, especially for patients ineligible for HSCT. Importantly, a recent microcosting analysis derived from the TRANSFORM study suggests that although liso-cel incurs higher upfront costs than stem cell transplants, it may reduce the need for subsequent therapies and downstream expenses, thereby supporting its long-term cost-effectiveness.49 Moreover, CAR T-cell therapy list prices often undergo reductions through confidential manufacturer-insurer negotiations, which are key to lowering treatment costs and shifting the economic scale toward cost-effectiveness.
Although our study focuses on the cost-effectiveness of second-line CAR T-cell therapy for DLBCL, alternative therapies are gaining traction. The combination of polatuzumab vedotin with bendamustine and rituximab, based on the phase 2 GO29365 study,50 reported a 40% CR and 9.5-month median PFS. Similarly, tafasitamab combined with lenalidomide, as seen in the phase 2 L-MIND study,51 recorded a 43% CR with a 12.1-month median PFS. However, direct comparisons between these therapies and CAR T-cell treatment are currently challenging because of variations in trial eligibility criteria and patient populations. For instance, L-MIND specifically excluded patients with primary refractory disease, a group that represented three-fourths of the TRANSFORM population. Additionally, patients with double-hit lymphoma, which constituted about a quarter of TRANSFORM population, were also excluded.
Our findings should be interpreted with these limitations in mind. The use of standard parametric survival modeling may not capture the full extent of long-term survival benefits, potentially overlooking any flattening of survival curves over time. The ongoing, extended follow-up in the TRANSFORM study is essential for more accurate efficacy estimates. In our study, the crossover of patients from SC to liso-cel could introduce confounding effects on the OS curve, especially considering the short follow-up period. Furthermore, our model, although aligned with clinical trial design, may not accurately reflect real-world scenarios. This includes the omission of HSCT after CAR T-cell therapy and the inability to incorporate novel agents used after SC due to data limitations. These gaps highlight the need for real-world evidence on subsequent therapies, which could greatly affect the perceived cost-effectiveness of CAR T-cell therapies. Finally, our analysis does not account for productivity losses after patient relapse, such as sick leave duration, return-to-work rates, or early retirement, due to incomplete data.
In conclusion, liso-cel demonstrates cost-effectiveness in treating patients with R/R DLBCL within the $100 000 per QALY willingness-to-pay threshold, particularly when analyzed over a lifetime horizon from both health care sector and societal perspectives. However, this cost-effectiveness is less certain under a 5-year horizon and when considering the therapy's list price increase from 2022 to 2023. The inclusion of broader societal costs suggests a potential economic advantage for CAR T-cell therapies, contingent on sustained clinical outcomes and corroborating real-world evidence. Our study contributes novel insights to the expanding research on the economic and societal implications of CAR T-cell therapies. It reaffirms the importance of evaluating CAR T-cell therapies beyond their immediate costs, emphasizing their long-term clinical and societal impacts for a more comprehensive assessment of their overall value.
Acknowledgments
The study is supported in part by the award number P30CA014089 (M.A.) from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. T.Y. reported receiving personal fees from AbbVie outside of submitted work.
Authorship
Contribution: J.H.C. and M.A. contributed to the study conceptualization; all authors reviewed the literature; J.H.C. performed the data collection, formal analysis, and visualization; J.H.C., T.Y., and M.A. contributed to the data curation and investigation; J.H.C. and M.A. wrote the draft manuscript; all the authors contributed to methodology, validation, and review and editing of the manuscript; and M.A. approved the final manuscript.
Conflict-of-interest disclosure: J.S.A. reported receipt of personal fees from Celgene, Incyte, Takeda, Janssen, Genentech, Eli Lilly, Kite Pharma, AbbVie, AstraZeneca, Epizyme, Genmab, Century Therapeutics, Regeneron, MorphoSys, BeiGene, Mustang Bio, Ono Pharma, Kymera, bluebird bio, C4 Therapeutics, Caribou BioSciences, Interius, Cellectar, and Bristol Myers Squibb; and receipt of grants from Bristol Myers Squibb, Seattle Genetics, Mustang Bio, Cellectis, and Merck. The remaining authors declare no competing financial interests.
Correspondence: Mohamed Abou-el-Enein, Division of Medical Oncology, Department of Medicine, Keck School of Medicine University of Southern California, 1450 Biggy St, Health Sciences Campus, Los Angeles, CA 90033; email: Mohamed.Abouelenein@med.usc.edu.
References
Author notes
Original data are available on request from the corresponding author, Mohamed Abou-el-Enein (mohamed.abouelenein@med.usc.edu).
The full-text version of this article contains a data supplement.