Key Points
Patients with limited (<5 disease sites) pre-CART B-cell NHL are at high risk of local relapse/progression.
BRT before CART for patients with R/R NHL and limited disease (<5 involved sites) improves outcomes without causing significant toxicities.
Visual Abstract
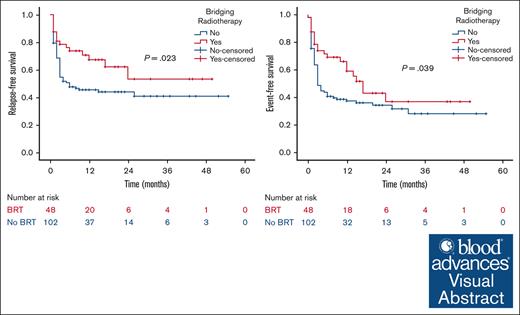
Unirradiated patients with relapsed/refractory (R/R) B-cell non-Hodgkin lymphoma (NHL) who undergo anti-CD19 chimeric antigen receptor T-cell therapy (CART) have a predominant localized pattern of relapse, the significance of which is heightened in individuals with limited/localized disease before CART. This study reports on the outcomes of patients with R/R NHL and limited (<5 involved sites) disease bridged with or without radiotherapy. A multicenter retrospective review of 150 patients with R/R NHL who received CART with <5 disease sites before leukapheresis was performed. Bridging treatment, if any, was administered between leukapheresis and CART infusion. Study end points included relapse-free survival (RFS), event-free survival (EFS), and overall survival. Before CART infusion, 48 patients (32%) received bridging radiotherapy (BRT), and 102 (68%) did not. The median follow-up was 21 months. After CART infusion, BRT patients had higher objective response (92% vs 78%; P = .046) and sustained complete response rates (54% vs 33%; P = .015). Local relapse in sites present before CART was lower in the BRT group (21% vs 46%; P = .003). BRT patients had improved 2-year RFS (53% vs 44%; P = .023) and 2-year EFS (37% vs 34%; P = .039) compared with patients who did not receive BRT. The impact of BRT was most prominent in patients who had ≤2 pre-CART involved disease sites, with 2-year RFS of 62% in patients who received BRT compared with 42% in those who did not (P = .002). BRT before CART for patients with limited (<5 involved disease sites) R/R NHL improves response rate, local control, RFS, and EFS without causing significant toxicities.
Introduction
Aggressive relapsed/refractory (R/R) B-cell non-Hodgkin lymphoma (NHL) remains a significant therapeutic challenge with poor outcomes. Anti-CD19–directed chimeric antigen receptor T-cell therapies (CARTs) are approved for the treatment of R/R B-cell NHL and has demonstrated an objective response rate of 46% to 86% and a complete response (CR) rate of 28% to 66% for refractory large B-cell subtype.1-5 However, sustained CART efficacy is limited, with durable CR rates of ∼30% to 40%.1,6 Most post-CART relapses have a local component (>80%) involving preexisting sites of disease present before CART infusion.7,8 Patients who progress after CART have poor overall survival (OS).9 Therefore, advances in CART development or augmentation of CART with synergistic treatments is warranted to improve on these outcomes.
Radiation therapy (RT) is a promising complement to CART because patients who receive CART usually have chemoresistant but radiosensitive disease.10 Bridging radiotherapy (BRT) before CART infusion has shown to be safe and effective with excellent local control (LC) rates >80%.11 It helps control the disease during the CART manufacturing period, improves rates of CART infusion, and, in retrospective series, augments response rates.7,12-19 Patient selection to identify those who benefit most from BRT remains an evolving process. Previous studies defined limited/oligometastatic peri-CART lymphoma as disease involving <5 sites and demonstrated improved outcomes for those with limited disease who received radiation in the bridging, consolidation, and salvage settings.11,20,21 Similarly, preautologous stem cell transplant RT has consistently shown to benefit patients with limited/localized relapsed pretransplant disease.22-24
Therefore, this study aims to report on the outcomes of patients with R/R B-cell NHL and limited (<5 involved sites) pre-CART disease, bridged with or without radiotherapy.
Methods
Imaging and disease burden
All patients had baseline positron emission tomography (PET)/computed tomography (CT) scan before leukapheresis. Ann Arbor staging system was used to define the number of involved disease sites. An involved disease site included the Ann Arbor anatomical lymph node region(s) harboring the PET-avid lymph node(s) or any PET-avid extranodal lesion. Only patients with limited disease, defined as <5 disease sites before leukapheresis were included. This number is based on our previous publications that defined peri-CART limited relapsed disease.11,20,21
Treatment characteristics
CART was a single infusion of either axicabtagene ciloleucel, tisagenlecleucel, brexucabtagene autoleucel, or lisocabtagene maraleucel on day 0. Bridging therapy (chemotherapy/immunotherapy or radiation) was defined as treatment administered between leukapheresis and CART infusion. Comprehensive BRT was defined as treating all PET-avid disease sites identified on the PET/CT scan before leukapheresis. Focal BRT was defined in cases in which not all disease sites were treated. RT was delivered through intensity-modulated radiotherapy, 3-dimensional radiotherapy, or spot-scanning proton therapy techniques. RT was at the discretion of the treating physician and consisted of PET-directed residual site radiotherapy targeting the CT anatomic mass with PET avidity only. The RT equivalent 2-Gy dose was calculated based on an alpha-to-beta ratio of 10.
Toxicity grading
Grading of cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS) were based on American Society for Transplantation and Cellular Therapy CRS consensus grading and immune effector cell–associated encephalopathy criteria, respectively.25 These scores were calculated prospectively after the patient received CART. Radiation side effects were graded prospectively per the Common Terminology Criteria for Adverse Events version 5 criteria during and at the end of radiation treatment.
Response assessment
After CART infusion, response to treatment (CART with or without BRT) was assessed on day +30 with a PET/CT scan using the Lugano criteria.26 Subsequent imaging was performed for the assessment of response or lack thereof.
Local relapse was defined as disease recurring at the same site(s) (with or without new sites) present on PET imaging before CART infusion. Distant relapse was defined as disease recurring outside the sites present on PET imaging before CART infusion.
In-field recurrence was defined as disease relapse occurring within the radiation planning target volume and was evaluated based on a careful review of the treatment plans that displayed the treatment fields/volumes and the PET-CT scans that identified the disease relapse.
Statistical methods and study end points
Statistical analysis was performed using IBM SPSS 28.0 software. Continuous data were reported as medians and ranges. Categorical data were reported as frequencies and percentages. Comparisons of different characteristics between the 2 groups were done using χ2 test. The nonparametric independent samples median test was used to compare median values between the 2 groups. LC, relapse-free survival (RFS), event-free survival (EFS), and OS were estimated by Kaplan-Meier survival curves. Survival differences were assessed by the log-rank test. Multivariate survival analyses were performed using Cox proportional hazards models (Cox regression). Univariate analysis accounted for BRT and the pre-CART baseline characteristics that may affect outcomes (age, sex, histology, size, maximum standardized uptake value, extranodal involvement, number of prior therapy lines, number of active lesions, and preleukapheresis levels of lactate dehydrogenase, C-reactive protein (CRP), and ferritin). Variables with a P value < .05 in the univariate analysis were included in a multivariate stepwise logistic analysis, and variables significant at P value < .05 were retained in the final model. The 95% confidence interval (95% CI) was calculated by the Wilson method. All reported P values were 2-sided, and differences were considered statistically significant at P values < .05.
The median follow-up period was calculated from the CART infusion date to the last documented follow-up visit. BRT LC was defined from the RT start date to in-field recurrence, in any of the irradiated sites, found on subsequent PET/CT scans. RFS was defined from CART infusion to any disease relapse/progression. EFS was defined from CART infusion to any disease relapse/progression, initiation of post-CART consolidative/salvage therapy, or death. OS was defined from CART infusion to death.
After institutional review board (IRB) approval, records of consecutive patients diagnosed with R/R B-cell NHL without bone marrow involvement by PET who received CART and had <5 disease sites before leukapheresis were retrospectively studied across 3 institutions between 2018 and 2023. IRB approval was granted (IRB number 20-006748).
Results
Management approach before CART infusion
A total of 150 patients with R/R B-cell NHL, who received CART and had <5 involved disease sites before leukapheresis, were identified. Before CART infusion, 48 patients (32%) received BRT, and 102 (68%) did not. Bridging systemic therapy was given to 5 (10%) and 36 patients (35%) in the BRT and no BRT groups, respectively (P = .001). Bridging systemic therapies consisted on lenalidomide (n = 1), ibrutinib (n = 1), methotrexate (MTX; n = 1), ARA-C–thiotepa (cytarabine combined with thiotepa; n = 1), DHAP (dexamethasone, high-dose cytarabine, and cisplatin; n = 1), RDHAP (rituximab, dexamethasone, high-dose cytarabine, and cisplatin; n = 1), Hyper-CVAD (cytarabine, cyclophosphamide, vincristine, and doxorubicin; n = 1), RGDP (rituximab, gemcitabine, dexamethasone, and cisplatin; n = 1), Rituxan (rituximab; n = 2), GEMOX (gemcitabine and oxaliplatin; n = 2), RGEMOX (rituximab, gemcitabine, and oxaliplatin; n = 3), RICE (rituximab, ifosfamide, carboplatin, and etoposide; n = 4), pola-R (polatuzumab vedotin and rituximab; n = 5), and pola-BR (polatuzumab vedotin, bendamustine, and rituximab; n = 16).
The median follow-up from CART infusion was 21 months for the entire cohort (20 months for the BRT group and 21 months for the no BRT group).
Baseline characteristics before CART infusion
The median age of the cohort was 57 years (range, 18-84), and the median number of involved disease sites before leukapheresis was 2 (range, 1-4). The median time between leukapheresis and CART infusion was longer in the BRT group (33.5 vs 28 days; P = .043). The female proportion and extranodal involvement were higher in the BRT group. Before leukapheresis, the level of CRP was significantly higher in the BRT group. However, there were no other significant differences in the remaining baseline clinicodemographic characteristics before CART infusion between the 2 groups. Further baseline disease-specific and patient-specific characteristics are listed in Table 1.
BRT and LC
The median equivalent 2 Gy (EQD2) dose for BRT was 25.5 Gy (range, 9.3-43), and the most common BRT regimen was 20 Gy in 5 fractions. The median time between BRT initiation and CART infusion was 29 days (range, 18-101). BRT was delivered through 3-dimensional radiotherapy (n = 26), intensity-modulated radiotherapy (n = 21), or proton therapy (n = 1) techniques. Comprehensive BRT was given to 37 patients to a median EQD2 of 28 Gy, and focal BRT was given to 11 patients to a median EQD2 of 23.3 Gy. Among the 48 BRT patients, 8 (16.7%) had in-field recurrences translating to a 2-year LC rate of 82%. In-field recurrences had a pretreatment median tumor size of 13 cm (range, 2-21), median maximum standardized uptake volume on PET scan (SUVmax) of 17.6 (range, 10-30.6), 4 of 8 extranodal involvement (50%), and were treated to a median EQD2 of 23 Gy. No significant correlation between BRT dose and in-field recurrence was found; however, among patients who underwent comprehensive BRT, there were no local failures with doses >30-Gy EQD2, resulting in a 100% LC rate. This contrasts with an 80% LC rate for those bridged with comprehensive BRT to doses of ≤30-Gy EQD2 (P = .076). Moreover, patients who received comprehensive BRT to all visible PET-avid disease sites (n = 37) had higher 2-year RFS (57% vs 45%; P = .1) and OS (66% vs 42%; P = .067) and significantly better 2-year EFS (44% vs 18%; P = .013) than patients who received focal BRT to some but not all visible disease sites (n = 11).
Toxicity
CRS of any grade occurred in 85% BRT patients and 80% of patients who did not receive BRT (P = .46). ICANS of any grade occurred in 35% BRT and 50% patients who did not receive BRT (P = .094). Specifically, the rate of grade ≥2 CRS was 42% in the BRT group and 40% in the no BRT group (P = .864), and the rate of grade ≥2 ICANS was 21% and 34% in the BRT and no BRT groups, respectively (P = .093). There were no observed grade ≥3 BRT-related toxicities.
Response rate
Objective response rate (CR and partial response) by PET 5-point scale, accounting for the best response achieved after CART but before consolidation/salvage or progression, was higher in the BRT group (92% vs 78%; P = .046). Detailed description of CR, partial response, and no response rates are listed in Table 1. Among the 30 BRT patients (63%) who had CR, 26 (54%) had sustained CR with no subsequent progression at a median follow-up of 20 months. In contrast, among the 55 patients (54%) who did not receive BRT and achieved CR, 34 (33%) had sustained CR with no subsequent progression at a median follow-up of 21 months.
Pattern of relapse
Crude rates of relapse/progression were lower in the BRT group (33% vs 56%; P = .01). Local relapse involving preexisting sites (with or without new sites) present before CART were lower in the BRT group (21% vs 46%; P = .003; Table 1). Twenty-five patients who did not receive BRT (24.5%) and 3 BRT patients (6%) had isolated local relapse only, in preexisting sites present before CART. Twenty-two patients who did not receive BRT (21.5%) and 7 BRT patients (14.5%) had local relapse in preexisting sites, present before CART, in addition to distant progression in new sites not present before CART. Ten patients who did not receive BRT (10%) and 6 BRT patients (12.5%) had isolated distant progression only, in new sites not present before CART.
Patients’ outcomes
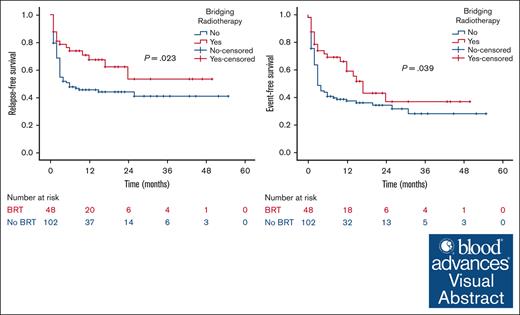
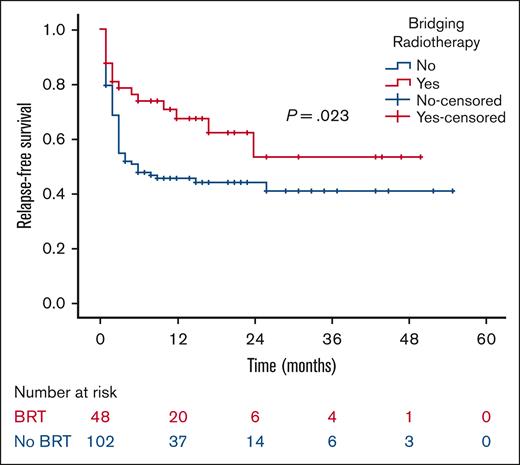
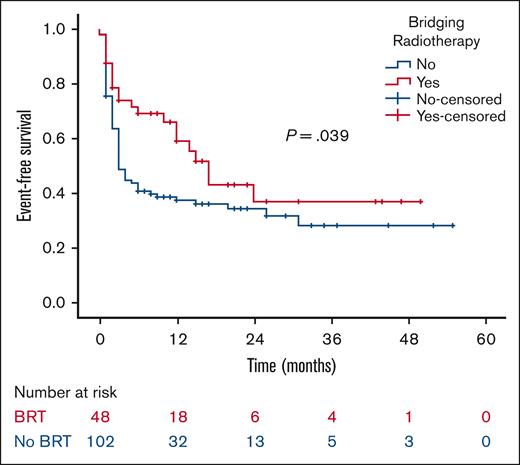
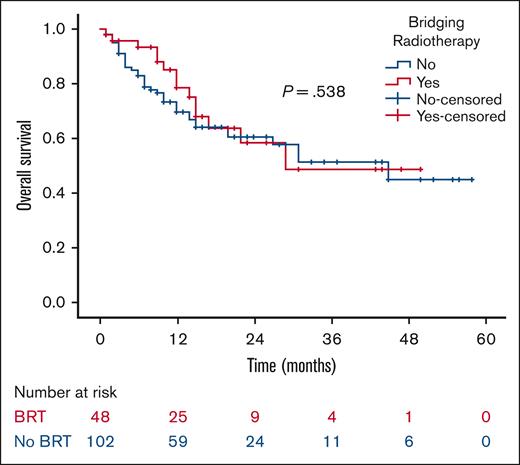
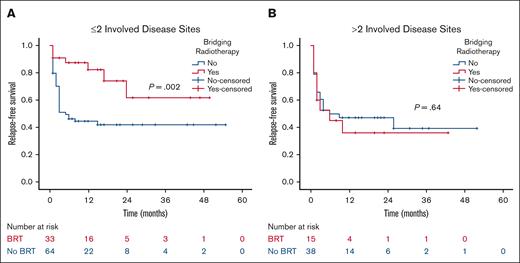
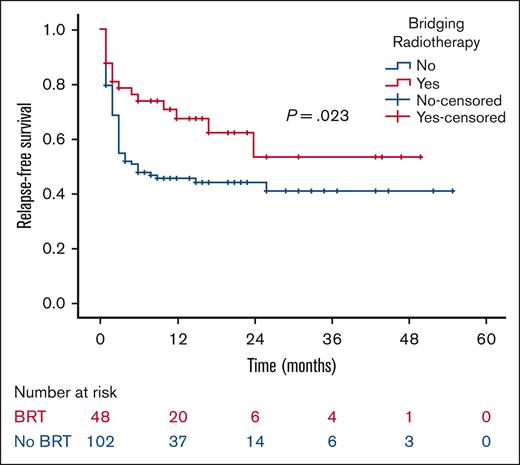
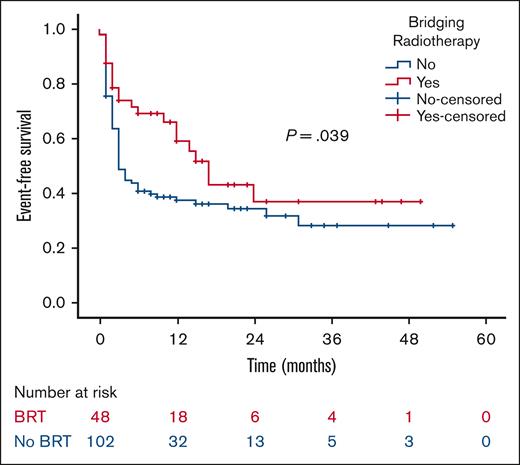
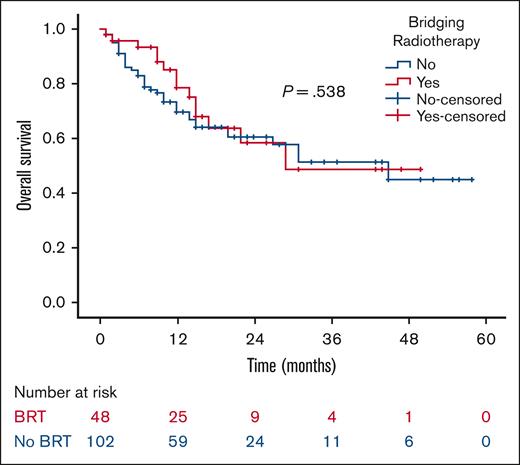
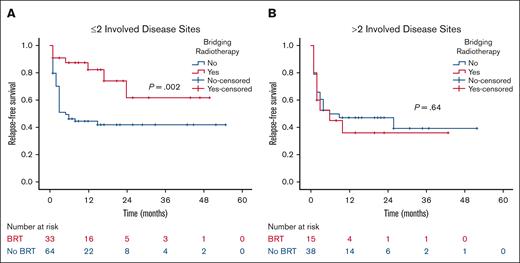
At a median follow-up of 21 months, the 2-year RFS was 53% in the BRT group and 44% in the no BRT group (P = .023; Figure 1). The 2-year EFS was 37% in the BRT group and 34% in the no BRT group (P = .039; Figure 2). The 2-year OS was 58% in the BRT group and 61% in the no BRT group (P = .538; Figure 3). The impact of BRT was most prominent in patients who had ≤2 pre-CART involved disease sites, with 2-year RFS of 62% in patients who received BRT compared with 42% in those who did not (P = .002). This improvement in RFS was not seen in patients who had >2 pre-CART involved disease sites (Figure 4).
RFS according to number of involved disease sites. RFS by receipt of BRT in patients with ≤2 (A) and >2 (B) involved disease sites.
RFS according to number of involved disease sites. RFS by receipt of BRT in patients with ≤2 (A) and >2 (B) involved disease sites.
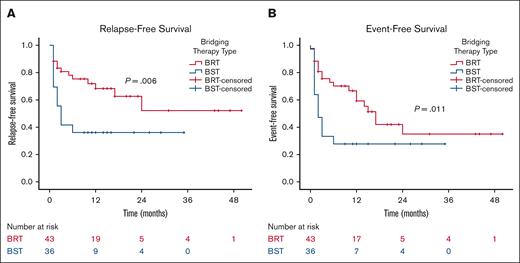
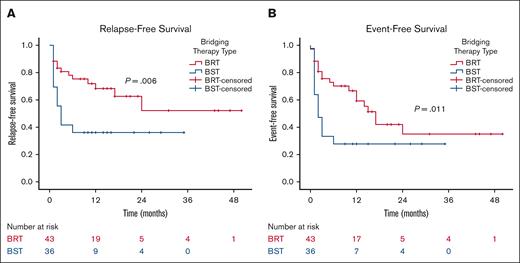
When patients are stratified based on the type of bridging therapy (BRT vs bridging chemotherapy/immunotherapy) and excluding those who received both bridging therapies, patients bridged with radiation (n = 43) demonstrated significant improvements in 2-year RFS (52% vs 36%; P = .006) and 2-year EFS (35% vs 28%; P = .011) compared with those bridged with chemotherapy/immunotherapy (n = 36; Figure 5). Off note, the pre-CART baseline characteristics between these 2 groups, accounting for the number of previous lines of therapy, tumor size, SUVmax, number of disease sites, extranodal involvement, lactate dehydrogenase, CRP, and ferritin levels, were not significantly different.
Outcomes according to type of bridging therapy. RFS (A) and EFS (B) by the type of bridging therapy. BST, bridging systemic therapy with chemotherapy/immunotherapy.
Outcomes according to type of bridging therapy. RFS (A) and EFS (B) by the type of bridging therapy. BST, bridging systemic therapy with chemotherapy/immunotherapy.
To further assess the association of various baseline/treatment characteristics with disease progression and prognosis, univariate analysis was conducted for all patients. BRT (hazard ratio [HR], 0.55; 95% CI, 0.32-0.96; P = .036), number of prior lines of systemic therapy (HR, 1.40; 95% CI, 1.12-1.80; P = .04), and preleukapheresis ferritin level (HR, 1.00; 95% CI, 1.000-1.001; P = .022) were significantly associated with RFS. On multivariate analysis, BRT (HR, 0.42; 95% CI, 0.23-0.80; P = .008) and the number of prior lines of systemic therapy (HR, 1.35; 95% CI, 1.06-1.72; P = .014) retained significant association with RFS, whereas preleukapheresis ferritin level (HR, 1.00; 95% CI, 1.000-1.001; P = .052) had borderline significant association with RFS.
Post-CART radiotherapy
Nine patients (2 in BRT and 7 in no BRT groups) received consolidative radiotherapy for day 30 PET-positive residual disease, with only 1 patient subsequently experiencing progression. Twenty-six patients (6 in BRT and 20 in no BRT groups) received salvage/palliative RT for post-CART relapsed disease. Although 27 patients who did not receive BRT (26.5%) received post-CART radiation, 39 patients who did not receive BRT (38%) remained without disease progression and did not receive radiation in the bridging, consolidation, or salvage/palliative setting.
Discussion
This study reports on the outcomes of patients with limited/localized pre-CART R/R B-cell NHL, with specific focus on the role of BRT in the context of limited disease. BRT was found to be an independent prognosticator of disease progression, and patients with <5 involved disease sites before leukapheresis who received BRT had significantly improved outcomes with minimal added toxicity compared with those who did not receive BRT. In our cohort, patients who did not receive BRT had a higher risk of disease progression with a predominant localized pattern of relapse. However, BRT before CART improved response rates and LC, which resulted in an altered pattern of relapse, as evident with the lower rates of local relapses in the BRT cohort. Such augmentation in response rates and LC for limited pre-CART disease translated to an improvement in RFS and EFS.
Several CART challenges warrant the use of bridging therapies. There is a significant amount of wait time from identifying the patient in need for CART to product infusion. Patients have to be medically cleared and approved by their insurer, followed by leukapheresis and ∼3- to 4-week manufacturing period. This time interval constitutes a major challenge, especially in the setting of rapidly progressive disease, because drop off rates ranging from 9% to 30% were observed in CART landmark trials1,2,27 owing mostly to disease progression. The presence of bulky and high-burden pre-CART disease has been associated with higher risk of local relapse and worse survival outcomes.8,28,29 Despite the promising objective and CR rates of CART, approximately two-thirds of patients relapse after CART, with durable CR rates of ∼30% at 5 years.30 Such relapse predominantly involves previous sites of disease (86%) identified before CART.7,8
Radiation stands as a promising bridging pre-CART treatment because it can help mitigate the aforementioned challenges. BRT can help control the disease during the CART manufacturing process and can increase the success rate of CART infusion. Lymphoma at this stage is chemoresistant but radiosensitive even to low doses of RT.10 Studies, including ours, comparing BRT with bridging systemic therapy have demonstrated inferiority of bridging systemic therapy,7,15,17 highlighting the importance of understanding the biology of the disease at this stage. BRT is effective in debulking/cytoreducing the lymphoma tumor burden,31 which can improve survival outcomes for patients with bulky disease,17 and results in comparable outcomes with patients with far lower disease burden who did not undergo irradiation.32 In our study, the similarity in CRP values before CART compared with the differing levels observed initially before leukapheresis may suggest that BRT played a role in either mitigating inflammation or preventing its exacerbation. Moreover, BRT is associated with excellent durable LC rate of ≥80% at 1 year,7,11,33 which can help overcome the localized pattern of post-CART relapses. The importance of LC becomes more important in patients presenting with high-risk features predictive of local relapse after CART, such as tumor size of ≥5 cm, SUVmax of ≥10, and extranodal involvement.8 BRT is strongly believed to help decrease the risk of local relapse for patients with high-risk features and potentially improve the outcome for this subset with localized/limited pre-CART disease.34 Similar benefit is seen in historical pre–stem cell transplant RT studies in lymphoma, which showed improved outcomes for patients with localized relapsed disease who received RT.22-24
Our study reports on the role of BRT for patients with limited pre-CART disease. It showed significant improvement in outcomes for patients who received BRT compared with those who did not, supporting the importance of local therapy for these patients in the setting of a predominant local pattern of relapse. Such improvement is more appreciated when all active disease is controlled with radiation (comprehensive BRT), which further decrease the risk of local relapse, which in the setting of localized/limited disease could translate into improved disease survival outcomes. These patients may be converted to CR with comprehensive BRT before CART infusion. Patients with complete metabolic response before CART achieve improved CART expansion that is correlated with improved outcomes.35 Although, in this study, we compared comprehensive with focal BRT and showed improved outcomes in the comprehensive BRT cohort, our previously published study further reinforces this concept by showing similar improvement with comprehensive BRT when compared with no bridging therapies (excluded focal BRT and bridging systemic therapy).21 Therefore, bridging therapies, specifically RT, can be valuable in getting rid of gross metabolically active disease. This may reduce CART exhaustion, giving them more space to fight microscopic disease and induce longer remission rates.
The role of local therapy becomes more relevant as we move forward in understanding and defining oligometastatic lymphoma. Previous RT and CART studies have shown improved outcomes with RT in the consolidation20,36 and salvage settings11,37 for patients with <5 involved disease sites. Similarly, our study reports improved outcomes for patients with <5 involved disease sites in the pre-CART/bridging setting, completing the circle of RT benefit for limited peri-CART lymphoma. From an oligometastatic standpoint, the impact of BRT in our cohort was mostly evident in patients with ≤2 involved disease sites. While acknowledging the lower number of patients with >2 disease sites in our study, the tumor burden, and the decreased feasibility of comprehensive BRT with increasing number of disease sites, one can further appreciate the importance of systemic therapies, such as CART, for patients with NHL who have multiple disease sites, predisposing them to greater risk of microscopic disease that might not be taken care of with local therapies. However, systemic therapy alone is not always sufficient for durable cure, even in low volume disease. Our study showed that >50% of patients with ≤2 involved pre-CART disease sites who did not get irradiated relapsed with inferior outcomes compared with those bridged with RT. Identifying patients who would benefit most from BRT remains a challenge worth further investigation, even in patients with limited pre-CART disease. Our study showed 38% of patients who did not receive BRT neither relapsed or required any bridging, consolidation, or salvage RT. Therefore, identifying patients with limited pre-CART disease who are at high risk of progression is important before adding synergistic treatments to CART. Nonetheless, the detrimental outcome of post-CART relapses calls for maximal utility of upfront peri-CART treatments, and BRT was highly effective and minimally toxic in our cohort.
This study is limited by its retrospective nature, sample size, and short follow-up period. RT was delivered at the discretion of the treating radiation oncologist, with no standardized dosing and fractionation regimens. There were no clear indications for referring patients to receive BRT, and the decision was at the discretion of the treating hematologist. Longer follow-up is needed to confirm the response durability to BRT. Furthermore, it is important to acknowledge that patients with 1 disease site pre-CART have less tumor burden and will fall under the comprehensive BRT subcohort by default if they receive RT. This may limit comparisons between focal and comprehensive RT subgroups if tumor burden is not accounted for. Finally, despite the absence of significant differences in baseline characteristics between both cohorts, there might be other unaccounted confounding factors. For example, tumor metabolic volume is an emerging prognostic predictor of prognosis that is not included in our study.
Conclusion
Patients with limited (<5 disease sites) pre-CART B-cell NHL are at high risk of local relapse. BRT before CART infusion for these patients appear to alter the pattern of relapse and improve response rate, LC, RFS, and EFS without significant toxicity. Larger prospective studies are needed to confirm our findings, and clinical predictors of which patients will benefit most from BRT are needed.
Authorship
Contribution: O.S., J.L.P., and B.S.H. contributed to conception and design; O.S., J.L.P., B.S.H., M.A.K.-D., Y.L., and W.G.B. were responsible for the provision of study materials or patients; O.S., W.G.B., R.B., J.L.P., B.S.H., and M.A.K.-D. contributed to the collection and assembly of data; O.S., J.L.P., B.S.H., and M.A.K.-D. performed data analysis and interpretation; and all authors contributed to manuscript writing, provided final approval of the manuscript, and are accountable for all aspects of the work.
Conflict-of-interest disclosure: J.M. reports consulting for Pharmacyclics/AbbVie, Bayer, Gilead/Kite Pharma, Pfizer, Janssen, Juno/Celgene, Bristol Myers Squibb (BMS), Kyowa Kirin, Alexion, BeiGene, Fosun Kite, Innovent, Seagen, Debiopharm, Karyopharm, Genmab, Antibody-Drug Conjugate Therapeutics, Epizyme, and Servier; received research funding from Bayer, Gilead/Kite Pharma, Celgene, Merck, Portola, Incyte, Genentech, Pharmacyclics, Seagen, Janssen, and Millennium; honoraria from Targeted Oncology, OncView, Curio, Kyowa Kirin, Physicians' Education Resource, and Seagen; and speakers' bureau fees from Gilead/Kite Pharma, Kyowa Kirin, Bayer, Pharmacyclics/Janssen, Seagen, Acrotech/Aurobindo, BeiGene, Verastem, AstraZeneca, Celgene/BMS, and Genentech/Roche. Y.L. reports consulting for Kite/Gilead, Celgene/BMS, Juno/BMS, bluebird bio, Janssen, Legend Biotech, Gamida Cells, Novartis, Iovance, Takeda, Fosun Kite, and Pfizer; received research funding from Kite/Gilead, Celgene/BMS, bluebird bio, Janssen, Legend Biotech, Merck, Takeda, and Boston Scientific; serves on data safety and monitoring board for Sorrento; is a member of data review committee for Pfizer; and serves on scientific advisory committee for NexImmune. H.S.M. reports advisory board fees from CRISPR Therapeutics, Senti Biosciences, and Jazz Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Jennifer L. Peterson, Department of Radiation Oncology, Mayo Clinic, 4500 San Pablo Rd S, Jacksonville, FL 32224; email: peterson.jennifer2@mayo.edu.
References
Author notes
All relevant data are available in the article, supplemental information can be obtained upon reasonable request from the corresponding author, Jennifer L. Peterson (peterson.jennifer2@mayo.edu).