Key Points
COVID-19 mRNA vaccination has a good risk-benefit profile in individuals with sickle cell disease.
This study offers some assurances to the sickle cell disease community and their providers about the safety and efficacy of mRNA vaccines.
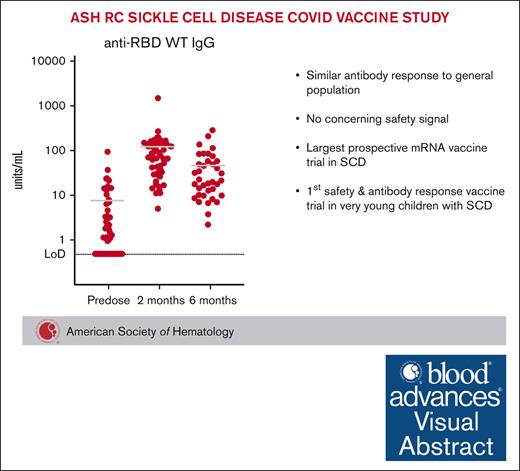
Visual Abstract
Children and adults with sickle cell disease (SCD) have increases in morbidity and mortality with COVID-19 infections. The American Society of Hematology Research Collaborative Sickle Cell Disease Research Network performed a prospective COVID-19 vaccine study to assess antibody responses and analyze whether messenger RNA (mRNA) vaccination precipitated any adverse effects unique to individuals with SCD. Forty-one participants received 2 doses of the Pfizer-BioNTech vaccine and provided baseline blood samples before vaccination and 2 months after the initial vaccination for analysis of immunoglobulin G (IgG) reactivity against the receptor binding domain (RBD) of the severe acute respiratory syndrome coronavirus 2 spike protein. Six-month IgG reactivity against the viral RBD was also available in 37 patients. Postvaccination reactogenicity was common and similar to the general population. There were no fevers that required inpatient admission. Vaso-occlusive pain within 2 to 3 days of first or second vaccination was reported by 5 participants (12%) including 4 (10%) who sought medical care. Twenty-seven participants (66%) were seropositive at baseline, and all 14 initially seronegative participants (34%) converted to seropositive after vaccination. Overall, mRNA vaccination had a good risk-benefit profile in individuals with SCD. This mRNA vaccine study also marks the first evaluation of vaccine safety and antibody response in very young children with SCD. This trial was registered at www.ClinicalTrials.gov as #NCT05139992.
Introduction
Sickle cell disease (SCD) has been called the most common severe genetic disease. It shortens life expectancy by at least 20 years and can induce multiorgan impairment owing to vascular occlusion, ischemia reperfusion injury, and acute and chronic inflammation. Individuals with SCD have an increased risk of developing infections owing to asplenia and impairment of the alternative complement pathway, humoral responses, and CD4 T-helper cells.1-5 The efficacies of vaccinations to induce antibodies against hepatitis B, Streptococcus pneumoniae, Haemophilus influenzae, and perhaps influenza can be impaired in children with SCD.6-9
Given that COVID-19 hospitalization rates are higher for individuals with SCD, COVID-19 messenger RNA (mRNA) vaccination is recommended.10,11 Concern has been raised about whether mRNA vaccines may induce unique disease-related complications in individuals with SCD. This uncertainty has led to apprehension that may have contributed to a suboptimal immunization rate among this patient population.8
As part of its initiative to improve the lives of individuals with SCD, the American Society of Hematology (ASH) Research Collaborative (RC) developed a Sickle Cell Disease Research Network to enable large scale collaborative research studies. Understanding the response to COVID-19 vaccination in this high-risk group of patients can provide a more targeted approach to vaccination, and an assessment of whether additional doses of vaccine will be required to achieve adequate protection. Knowledge of the immune response to COVID-19 mRNA vaccines may also provide useful information about the immune response to other mRNA vaccines as they become available. To address whether COVID-19 mRNA vaccination induces a normal immune response or precipitates unique toxicities in individuals with SCD such as vaso-occlusive events, the ASH RC Sickle Cell Disease Research Network performed a prospective clinical study.
Methods
This prospective, observational trial was performed under the framework of the ASH RC’s Sickle Cell Disease Research Network and was entirely funded by ASH. It was approved by the Western Copernicus Group Institutional Review Board (IRB) and is registered at ClinicalTrials.gov (NCT05139992). Eligible participants had SCD (any genotype), had not been previously vaccinated against COVID-19, and agreed to be vaccinated as part of routine clinical care. Western IRB was the central IRB.
Laboratory analysis
We measured anti–receptor binding domain (RBD) (Wuhan-Hu-1 strain) immunoglobulin G (IgG) concentrations by enzyme-linked immunosorbent assay in duplicate on plasma biospecimens obtained at baseline, 2 months, and 6 months after the initial vaccination.12,13 A control monoclonal antibody was included on each plate to standardize values. All collected plasma samples were frozen at the study institutions for batch shipments to the reference laboratory, and all samples were analyzed simultaneously. We reported the average of the 2 IgG results in arbitrary units. Based on our earlier study, samples with IgG levels >0.48 units per mL were considered seropositive; those with IgG ≤0.48 units per mL were deemed seronegative, indicating that no RBD antibodies were present.12
Statistical plan
It is acknowledged that vaccines against other diseases established specific antibody titers that provide protection against infection. Such guidelines are not available for COVID-19; therefore, antibody titers are presented descriptively. We calculated an initial accrual goal of 200 participants in December 2020, just before COVID-19 vaccination became available. Unfortunately, the study did not fully open for accrual until the first half of 2022, when many in the community had already been vaccinated.
Results and discussion
Eight ASH RC Sickle Cell Disease Research Network Consortia (led by investigators at Johns Hopkins, Prisma Health, Duke, UT Southwestern, Medical College of Wisconsin, UCSF Benioff Children's Hospital, Children’s National Medical Center, and Children’s Hospital at Montefiore) and an academic research organization (led by investigators at the University of Pennsylvania) participated in this study. Fifty-nine participants were enrolled between December 2021 and February 2023, 47 (80%) of whom received at least 1 COVID-19 vaccine dose. The 41 participants (69%) who contributed paired baseline and 2-month postinitial vaccine samples for analysis all received the Pfizer-BioNTech vaccine.
Study population
A total of 41 paired baseline and 2-month postinitial vaccination samples were provided for analysis of anti-RBD IgG from 17 adult participants (41%) and 24 pediatric participants (59%). Seven participants (17% of total study population) were younger than 3 years. Thirty participants (73%) had hemoglobin SS (HbSS), 9 (22%) had hemoglobin SC (Hgb SC), 1 (2%) had hemoglobin S beta+ (HbS beta+) thalassemia, and 1 (2%) had hemoglobin S beta0 (HbS beta0) thalassemia. Twenty-four participants (59%) were taking hydroxyurea at the time of enrollment, 7 (17%) were taking voxelotor, and 1 (2%) was taking crizanlizumab. Twelve participants (29%) reported that they had a COVID-19 infection before enrollment in this study. Six-month postvaccination samples were obtained from 37 of these 41 participants (90%) and analyzed in a separate batch run.
Vaccine side effects and SCD-associated symptoms
We used structured telephone interviews to query participants about the type of vaccine received, side effects, and sickle cell–related events 2 to 3 days after each vaccine dose and 2 weeks and 2 months after the initial vaccination. Adverse outcomes are presented in Table 1. Twenty participants (49%) reported at least 1 postvaccination side effect or SCD-related complication. The most common side effects were vaccine site pain and myalgia. No participants reporting fever were seen in a clinic or emergency department or admitted to a hospital for fever. Five participants (12.2%) reported vaso-occlusive pain in the 2 to 3 days after vaccine dosing, 4 (9.8%) needed additional pain medication, 3 (7.3%) sought medical care, and 1 (2.4%) was admitted to the hospital. All 5 individuals with vaso-occlusive pain had the Hgb SS genotype. There were no cases of splenic sequestration, aplastic crises, sepsis, or stroke during the 2-month postvaccination period. However, 3 participants (7.3%) were diagnosed as having acute chest syndrome 10 days, 42 days, or 43 days after vaccination. No participants reported cases of venous thromboembolism. Six additional subjects received at least 1 vaccination but did not submit a sample after vaccination and were therefore not included in the statistical analysis. However, none of these 6 subjects reported vaso-occlusive pain after vaccination.
Laboratory results
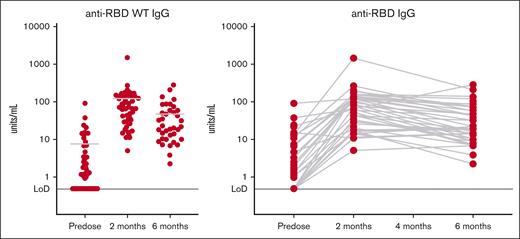
Anti-RBD antibody titers are shown in Figure 1. Fourteen participants (34%) were seronegative at baseline; the remaining 27 participants (66%) were seropositive (even though only 29% reported previous COVID-19 infection). All participants were seropositive at the 2-month postvaccination time point, which for 90% of the participants was between 54 to 76 days after the initial vaccination. After vaccination, the median IgG was 32.155 units per mL (interquartile range [IQR] 16.9-65; range, 4.99-168.04).
Antibody responses from participants. Shown are anti-RBD antibody titers expressed as the mean values of the participants (left) or by individual over time (right). Levels <0.48 units per mL are considered seronegative in healthy volunteers.13 Fourteen participants (34%) were seronegative at baseline. Twenty-seven participants (66%) were seropositive at baseline. All initially seronegative participants converted to seropositive after vaccination, although postvaccination IgG levels were lower than in the participants who were initially seropositive (Wilcoxon signed rank sum test, P = .0062).
Antibody responses from participants. Shown are anti-RBD antibody titers expressed as the mean values of the participants (left) or by individual over time (right). Levels <0.48 units per mL are considered seronegative in healthy volunteers.13 Fourteen participants (34%) were seronegative at baseline. Twenty-seven participants (66%) were seropositive at baseline. All initially seronegative participants converted to seropositive after vaccination, although postvaccination IgG levels were lower than in the participants who were initially seropositive (Wilcoxon signed rank sum test, P = .0062).
The 27 participants who were seropositive at baseline had a median baseline IgG of 4.59 units per mL (IQR, 1.81-14.37; range, 0.96-90.48). After vaccination, these individuals had a median IgG of 102.79 units per mL (IQR, 45.08-157.36; range, 11.4-1492.07). In the seropositive participants, the median fold change in IgG was 19.5 (IQR, 6.17-51.58; range, 0.74-176.02). The immune response in children younger than 3 years (including a 1-year-old) was similar. Although all participants who were seronegative at baseline did convert to seropositive after vaccination, the postvaccination IgG levels in the seronegative group remained lower than those in the group that was initially seropositive (P = .0062).
The summary statistics for the 37 individuals with 6-month data are presented in Table 2. To provide a comparator, we present the 2-month data for these 37 individuals, so the serologies over time can be compared. Given that titer of lower than 0.48 units/mL is the limit below which participants are considered seronegative, all other participants were seropositive. At both 2 and 6 months from initial vaccination, all participants who were measured were seropositive. The 6-month titers are lower than the 2-month titers (P < .0001). The median ratio of 6-month to 2-month titers was 0.4066, so the typical drop in titer was 60%. In 25% of the participants, the drop in titers was 80% or more between 2 months and 6 months after vaccination but remained above the seronegative level.
Final comments
The ASH RC’s Sickle Cell Disease Research Network performed this trial to assess the efficacy of the COVID-19 mRNA vaccine to induce immune response, and to determine whether this type of vaccine platform would induce vaso-occlusive events or other unique toxicities in individuals with SCD.
We observed that individuals with SCD mount antibodies against the RBD of severe acute respiratory syndrome coronavirus 2 roughly comparable with the general population and had a decline in circulating anti-RBD antibodies that was also similar to the immune decay that has been reported to occur in COVID-19–naïve and COVID-19–exposed vaccine recipients.14-16 It is notable that 27 participants (66%) had evidence of a severe acute respiratory syndrome coronavirus 2 infection before their initial vaccination. Side effects from the mRNA vaccines were common but typically mild.
To our knowledge, this study is the largest prospective study of COVID-19 mRNA vaccination of individuals with SCD and the only trial to show the rate of antibody decay over subsequent months. It is also the first to include very young children. We find it reassuring that data from smaller, briefer, and/or retrospective studies of individuals with SCD receiving mRNA vaccines or adenovirus based vaccines confirm the favorable efficacy and safety profile of COVID mRNA vaccines.17-21 It is our hope that this information will help inform providers and future research studies and offer some assurance to those in the SCD community that future mRNA vaccines can offer more benefit than risk to this patient population.
Authorship
Contribution: A.R.A., J.J.S., D.M., A.M.B., E.V., A.C., P.J.L., A.N., I.F.I., and J.J.F. performed research and wrote the manuscript; A.B. designed and coordinated the research; H.S.-C. and L.V. coordinated the research; Y.Z. and J.J.S.S. analyzed data; and S.E.H., N.M., S.L., D.N., and C.S.A. designed the research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: A.R.A. is a Pfizer consultant and member of the speakers bureau. J.J.S. is a medical expert for Health Resources and Services Administration Vaccine Injury Compensation Program. D.M. is a Pfizer consultant. A.M.B. is an adjudication board member for a Pfizer sponsored trial. A.C. is a recipient of grant funding from Pfizer. A.N. is a consultant for Pfizer. S.E.H. is a consultant for Pfizer. N.M. is a consultant for ASH Research Collaborative. S.L. is a consultant for Pfizer. The remaining authors declare no competing financial interests.
A complete list of the members of the investigators of the American Society of Hematology Research Collaborative Sickle Cell Disease Research Network appears in “Appendix.”
Correspondence: Sophie Lanzkron, Division of Hematology, Thomas Jefferson University, 1015 Chestnut St, Philadelphia, PA 19107; email: sophie.lanzkron@jefferson.edu; Donna Neuberg, Department of Data Science, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; email: neuberg@ds.dfci.harvard.edu; and Charles S. Abrams, Department of Medicine, University of Pennsylvania, 3400 Civic Center Blvd, Philadelphia, PA 19104; email: abrams@upenn.edu.
Appendix
The members of the American Society of Hematology Research Collaborative Sickle Cell Disease Research Network are Alan R. Anderson (Prisma Health Children’s Hospital – Upstate); Biree Andemariam (University of Connecticut Health); Amanda Brandow (Medical College of Wisconsin); Andrew Campbell (Children's National Medical Center); Alice Cohen (Newark Beth Israel Medical Center); Deepika Darbari (Children's National Medical Center); Fuad El Rassi (Emory University School of Medicine); Joshua Field (Medical College of Wisconsin); Ellen Fung (UCSF Benioff Children’s Hospital of Oakland); Beatrice Gee (Emory University School of Medicine); Ibrahim Ibrahim (University of Texas Southwestern Medical Center); Modupe Idowu (Texas Children’s Hospital/Baylor College of Medicine); Julie Kanter (University of Alabama at Birmingham); Elizabeth S. Klings (Boston Medical Center); Allison King (Washington University); Abdullah Kutlar (Augusta University Research Institute); Jeffrey D. Lebensburger (University of Alabama at Birmingham); Patrick Leavey (University of Texas Southwestern Medical Center); Robert I. Liem (Ann and Robert H. Lurie Children’s Hospital of Chicago); Deepa Manwani (Montefiore Medical Center); Shalu Narang (Newark Beth Israel Medical Center); Betty Pace (Augusta University Research Institute); Charles T. Quinn (Cincinnati Children’s Hospital Medical Center); Kenneth Rivlin (Jacobi Medical Center affiliated to Albert Einstein College); John J. Strouse (Duke University); Alexis A. Thompson (Children’s Hospital of Philadelphia); Venée N. Tubman (Texas Children’s Hospital/Baylor College of Medicine); Elliot Vichinsky (UCSF Benioff Children’s Hospital of Oakland); and Mark Walters (UCSF Benioff Children’s Hospital of Oakland).
References
Author notes
A.R.A., J.J.S., and D.M. contributed equally to this work.
Data are available on request from the corresponding authors, Sophie Lanzkron (sophie.lanzkron@jefferson.edu), Donna Neuberg (neuberg@ds.dfci.harvard.edu), and Charles S. Abrams (abrams@upenn.edu).