Key Points
In centers with robust clinical trial infrastructure and a strong culture of trials, clinical trial enrollment is high across demographics.
In our cohort, race, sex, and socioeconomic status were not predictors of trial participation, whereas age and diagnosis were predictive.
Visual Abstract
Prior studies have demonstrated that certain populations including older patients, racial/ethnic minority groups, and women are underrepresented in clinical trials. We performed a retrospective analysis of patients with non-Hodgkin lymphoma (NHL) seen at MD Anderson Cancer Center (MDACC) to investigate the association between trial participation, race/ethnicity, travel distance, and neighborhood socioeconomic status (nSES). Using patient addresses, we ascertained nSES variables on educational attainment, income, poverty, racial composition, and housing at the census tract (CT) level. We also performed geospatial analysis to determine the geographic distribution of clinical trial participants and distance from patient residence to MDACC. We examined 3146 consecutive adult patients with NHL seen between January 2017 and December 2020. The study cohort was predominantly male and non-Hispanic White (NHW). The most common insurance types were private insurance and Medicare; only 1.1% of patients had Medicaid. There was a high overall participation rate of 30.5%, with 20.9% enrolled in therapeutic trials. In univariate analyses, lower participation rates were associated with lower nSES including higher poverty rates and living in crowded households. Racial composition of CT was not associated with differences in trial participation. In multivariable analysis, trial participation varied significantly by histology, and participation declined nonlinearly with age in the overall, follicular lymphoma, and diffuse large B-cell lymphoma (DLBCL) models. In the DLBCL subset, Hispanic patients had lower odds of participation than White patients (odds ratio, 0.36; 95% confidence interval, 0.21-0.62; P = .001). In our large academic cohort, race, sex, insurance type, and nSES were not associated with trial participation, whereas age and diagnosis were.
Introduction
Non-Hodgkin lymphoma (NHL) is the seventh most common cancer and the ninth leading cause of cancer death for both men and women in the United States. In 2023, an estimated 80 550 people in the United States will be diagnosed with NHL, and 20 180 will die from NHL.1 Although the incidence of NHL in the United States has decreased and survival has improved in recent years,2 disparities on the basis of race, sex, age, insurance status, demographic location, socioeconomic status (SES), and other sociodemographic characteristics remain.3-9 There have been tremendous advancements in NHL therapies and outcomes in large part due to clinical trials, which are critical for advancing our understanding of cancer biology, evaluating the efficacy of novel treatments, and informing treatment decisions. Older individuals, certain minority groups (specifically Black and Hispanic patients), and women are recognized to be underrepresented in oncology clinical trials.10,11 A recent analysis of randomized controlled trials, which were crucial for the US Food and Drug Administration (FDA) approval of drugs for lymphoma treatment, has highlighted significant disparities in participant representation. Black patients, who make up 10.7% of NHL cases according to Surveillance, Epidemiology, and End Results Program statistics, were only 2.8% of the participants in these trials. Similarly, Hispanic patients, despite constituting 17.1% of patients with NHL, were underrepresented at 5.4% in these clinical trials.12 Underrepresentation of these groups in trials is detrimental to both these patient populations who do not receive equal access to novel therapies and to the trials that evaluate new approaches in participants who do not accurately reflect the patient populations in which the interventions are intended to be applied. In response to these findings, the US FDA now requires those seeking approval for late-stage clinical trials to submit a plan to ensure diversity among trial participants.13
Understanding the influence of social context on clinical trial enrollment is vital to identify potential areas in which tailored interventions can effectively address participation barriers as well as identify patient populations at risk of underrepresentation. Individual level data on socioeconomic factors such as household income, educational attainment, and employment status are not prospectively collected and/or consistently documented, making it difficult to assess the effects of these variables on health outcomes and health care utilization. Neighborhood-level socioeconomic measures based on patient addresses may approximate individual level effects while also capturing contextual effects of an individual’s environment.6,9,14 The objective of our study was to investigate the associations between clinical trial participation patterns, patient demographics, and neighborhood socioeconomic status (nSES) factors determined by place of residence among patients with NHL seen at the University of Texas MD Anderson Cancer Center (MDACC) located in Houston, Texas. The Department of Lymphoma and Myeloma at MDACC is one of the largest multidisciplinary programs for lymphoma and myeloma with a robust portfolio of lymphoma clinic trials as well as programs to support clinical trial enrolment, such as dedicated research nurse coordinators, patient navigators, and financial assistance programs. Although this study reflects the practice of a large single center, this is, to our knowledge, the first study to apply this approach for a comprehensive evaluation of clinical enrolment in patients with NHL across subtypes.
Methods
Data source and study populations
We performed a retrospective cohort study of adult patients with NHL seen in The MDACC Department of Lymphoma and Myeloma in Houston, Texas. Our cohort consisted of patients aged >18 years who had a first visit at MDACC between January 2017 and December 2020. All patients without verified NHL histology were excluded. We used an MDACC cancer registry that records consecutive patients with newly diagnosed invasive cancer identified at our institution and the department Lymphoma Outcomes Database (LOD) that records all patients with lymphoma seen at MDACC. The LOD includes data extracted directly from patient charts with quality assurance checks by trained data abstractors. We identified patients who participated in clinical trials based on clinical trial enrollment tracked for the Cancer Center Support Grant report.
Outcomes and clinical trials definitions
The primary outcome of this study was the clinical trial participation, defined as enrollment and participation in any clinical trial including both interventional and noninterventional studies, from January 2017 to December 2020.
Clinical trials were any investigational studies performed prospectively involving human participants. Clinical trials are broadly categorized as interventional or noninterventional. Noninterventional trials were largely observational and involved no prospective intervention or alteration in the status of the participants. Interventional trials were trials in which participants were prospectively assigned to receive a specific intervention. This assignment could have been random for some studies. Participants received diagnostic, treatment, behavioral, or other types of interventions. Interventional therapeutic trials involved specific therapies or procedures with therapeutic intent. Interventional nontherapeutic trials involved interventions without a specific therapeutic purpose, including diagnostic imaging or tissue sampling performed without therapeutic components.
Demographics and neighborhood socioeconomic variables
Patient demographic information at the time of diagnosis was obtained via the MDACC Epic electronic heath record (EHR) system.15 These data included age, sex, insurance status (uninsured, Medicare, Medicaid, and private insurance), and self-reported race (Black, White, Asian, American Indian/Alaska Native, Native Hawaiian or other Pacific Islander, and other/unknown) and ethnicity (Hispanic or non-Hispanic), with categories following the US Census Bureau standards. We used the Census Geocoder available through the US Census Bureau website to geocode the last known patient addresses to obtain the corresponding census tracts using the Master Address File/Topologically Integrated Geographic Encoding and Referencing database.16,17 Based on the census tracts, we then extracted 32 nSES variables (listed in supplemental Tables) from the American Community Survey (2015-2020) and 2020 decennial census data.18,19 These variables were selected based on those previously described20,21 and those used in prior nSES indices such as the area deprivation index (ADI).14,22 The nSES variables represent domains such as educational attainment, neighborhood median income, poverty, crowding, race composition, home values, and essential home utilities.
In addition to the individual nSES variables, we also used ADI as a composite measure of neighborhood socioeconomic disadvantage. The ADI is a validated percentile rank of socioeconomic disadvantage determined at the census block group level that has been used in numerous studies.23-26 ADI is calculated using 17 indicators related to income level, income disparity, educational attainment, employment, and home values. Census block groups were ranked on the percentile scale (1-100) based on their deprivation relative to the national level, with higher ADI scores indicating higher deprivation. These rankings are published by the University of Wisconsin School of Medicine and Public Health.26 We categorized ADI scores into quartiles to facilitate interpretation.
Geospatial analysis and travel distance
Using Google Maps Geocoding Application Program Interface (API), we obtained geographic coordinates (longitude/latitude) of patient residential addresses. For patients residing in Texas, the R package “googleway” was used to interface with the Google Direction API to calculate driving distance (km) and time (hours) from patients' residence to MDACC at the Texas Medical Center (TMC).27 To visualize the geographic distribution of patient population, we aggregated the number of participants by county and ZIP code tabulated area (ZCTA) and created choropleth maps using “tmap” R package.28 Similarly, choropleth maps were also generated to visualize and compare the distributions of patients with no trial participation, nonintervention trial participation, and therapeutic trial participation. We chose ZCTA as the spatial unit of visualization for the protection of patient data privacy.
Statistical analysis
We performed descriptive analysis to examine the distribution of demographic variables for the total population and those enrolled in clinical trials. We performed univariate analysis using the χ2 test for categorical variables and a 2-sample t test for continuous variables to assess the differences in clinical trial enrollment patterns by sex, race, age, histology type, insurance type, nSES variables, and driving distance to MDACC TMC. To address potential nonnormality in our data, we included the Mann-Whitney test for continuous variables, which is a nonparametric test and robust to skewed distributions. After a consideration of variables of interest and independence among them, we modeled the likelihood of clinical trial participation using generalized additive logistic regression. This model considered various factors such as LOD diagnosis group, age, sex, race, ADI national rank (quartiles), and census tract level education attainment (percentage of people aged ≥25 years with less than a high school education). The model included a penalized spline to accommodate the nonlinear association between participation and age. In assessing the differences across discrete variables, we used Dunnett-adjusted contrasts, referencing specific categories for comparison. An expanded model that included interaction between age and ADI rank quantile was explored but ruled out due to worse Akaike Information Criterion. We applied the same analytical procedure to subgroups with diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL). To account for multiple testing of the same data among these models, we used adjusted alpha of 0.05/3=0.017 as the criteria for significance in these models. All analyses were performed using R statistical software version 4.2.1.29
Institutional Review Board approval obtained for this study denoted ICD2022-0443.
Results
Patient characteristics and clinical trial participation patterns
A total of 3448 patients met the inclusion criteria of age ≥18 years with a histologically confirmed diagnosis of NHL treated at MDACC between January 2017 and December 2020. Of the patients identified, 130 had international addresses, and 172 did not have complete address information to be assigned to a census tract and were thus excluded from the final analysis. Therefore, the final cohort consisted of 3146 patients (Table 1). To assess for inadvertent selection bias, we compared the baseline characteristics between the final cohort and those excluded due to incomplete address information. No significant difference was detected, except in age (median age is 65 years for the study population and 68 years for the excluded patients; P < .0001). There was also a notable difference in the types of insurance between the study cohort and the excluded group; however, private insurance and Medicare were predominant in >90% of both cohorts. Importantly, overall clinical trial enrollment rate and the types of trials participated were comparable between the excluded patients and the final study cohort, as summarized in Table 2.
The study population consisted of 59.5% male and 74.9% non-Hispanic white (NHW), with 13.1% Hispanic, 6% Black, and 3.7% Asian patients. The most prevalent histology groups were DLBCL at 29.1%, FL at 22.2%, mantle cell at 13.9%, marginal zone at 9.1%, T-cell at 8.2%, and high-grade B-cell lymphoma at 6.5%. The most common insurance type was managed care (private preferred provider organization [PPO]/health maintenance organization [HMO] plans) at 47.8%, and Medicare at 46.2%; 2.5% of patients were self-pay, 1.1% had Medicaid, and 2.4% had other government insurance.
Within our cohort of 3146 patients, a subset of 1819 (58%) resided in Texas. The analysis of geographical distribution revealed that a substantial proportion of the patients were from Harris County (n = 558 [30.6%]), where the city of Houston is located (Figure 1A). Furthermore, the map of ZCTA sample size in Harris County (Figure 1B) indicated that most patients were from suburban areas, with an average driving distance of 34.3 km and travel time of 30.2 minutes to the medical center. Additionally, the geographic patterns of patients’ residencies differed in relation to the types of trials in which patients participated, as depicted in Figure 1C-F. This suggests that trial participation may correlate with geographic factors.
Geospatial distribution of patients’ residencies by trial participation. (A) Distribution of all patients within Texas. (B) All patients proximate to TMC. (C) Patients with any trial participation. (D) Patients with no trial participation. (E) Patients in noninterventional trials (with overlap). (F) Patients in therapeuric trials.
Geospatial distribution of patients’ residencies by trial participation. (A) Distribution of all patients within Texas. (B) All patients proximate to TMC. (C) Patients with any trial participation. (D) Patients with no trial participation. (E) Patients in noninterventional trials (with overlap). (F) Patients in therapeuric trials.
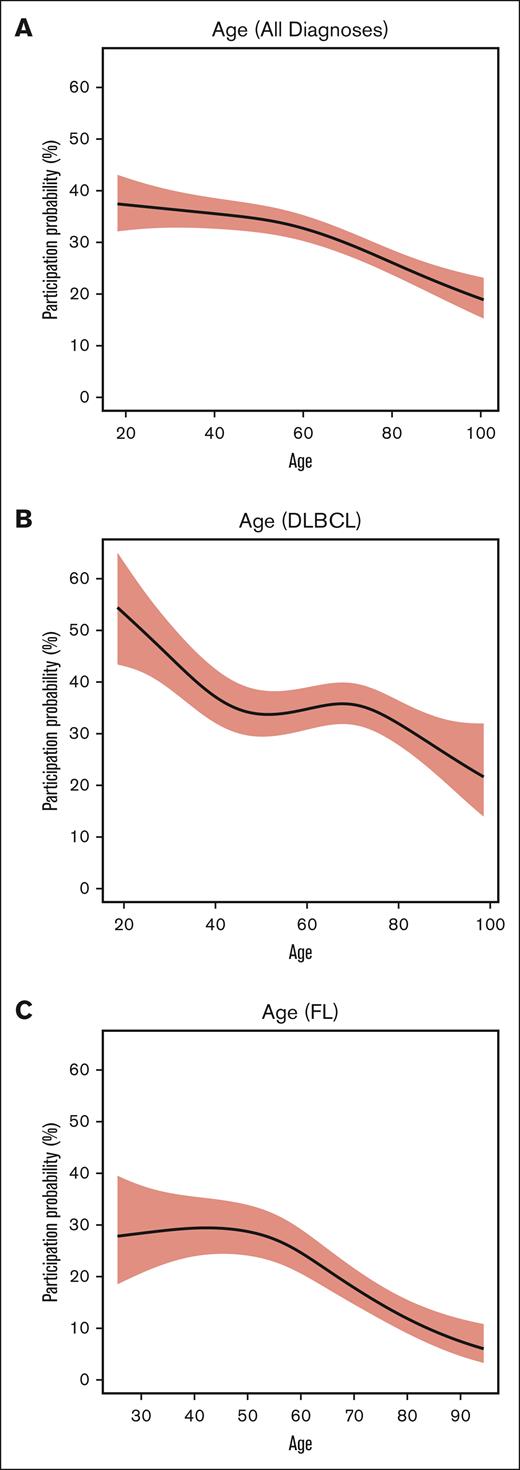
Depiction of trial participation by age. (A) Entire study cohort. (B) DLBCL subcohort. (C) FL subcohort.
Depiction of trial participation by age. (A) Entire study cohort. (B) DLBCL subcohort. (C) FL subcohort.
Characteristics of clinical trial participants and geospatial and univariate analysis
Clinical trial participants were 63.4% male, 78.1% NHW, 11.6% Hispanic, 5.3% Black, and 3.1% Asian. Clinical trial participants were slightly younger than nonparticipants (median age, 63 vs 65 years; P = .001). There was a high overall clinical trial participation rate of 30.3%, with the majority of patients being enrolled in therapeutic trials (20.9% of the study cohort). Patients also participated in noninterventional (12.7%) and interventional nontherapeutic (2.9%; Table 2). Many patients participated in >1 type of trial; thus, there was notable overlap predominantly between noninterventional and interventional therapeutic trials. There were higher participation rates among men than women (32.2% vs 27.6%; odds ratio [OR], 1.26; P = .006). In the total cohort, participation rates were similar across race/ethnicity. Most patients had managed care (private HMO/PPO plans; 47%) or Medicare (45.7%), and few patients had Medicaid (1.2%) or other government insurance. Participation rates did not differ by insurance type in this cohort. There was significant variation in clinical trial participation by histology type (P = .0005). The trial participation rates for DLBCL and FL were 28.2% and 22%, respectively. The highest trial participation rates were observed among patients with mantle cell lymphoma (54.6%) and T-cell lymphoma (39.4%), which contrasted with the lower rates among patients with marginal zone lymphoma (19.2%) and other types (10%).
In univariate analyses, 3 of the 31 nSES variables showed a significant association with clinical trial participation. Specifically, lower participation rates were correlated with areas with a higher percentage of the overall populations living below the poverty level (P = .007), a higher percent of populations aged 19 to 64 years living below the poverty level (P = .008), and a higher percent of populations living in crowded households (>1 person per room; P = .002). Further analysis using the Mann-Whitney test to address skewed data identified other significant associations. There are lower participation rates in areas with a higher percentage of the population aged >25 years with less than a high school diploma (P = .018), a lower percentage of the population aged >25 years with a bachelor’s degree (P = .048), a lower median household income (P = .047), a lower percentage of households with incomes above $200 000 (P = .004), and lower home values (P = .003). Moreover, the association between participation and ADI was significant (P = .043), and even stronger when analyzing only the participants in interventional/therapeutic trials (P = .007), as summarized in Table 2 and Table 3. Notably, there was no significant association between the racial composition of each census tract and clinical trial participation. There was also no significant association between clinical trial participation and either driving distance (OR, 1.23; confidence interval [CI], 0.83-1.84; P = .31) or driving time (OR, 1.03; CI, 0.94-1.14; P = .52).
Multivariable analysis
Multivariable analysis revealed a nonlinear inverse correlation between increasing age and the likelihood of clinical trial participation (P = .001), indicating the odds of clinical trial participation decrease as age increases (Figures 1-2). Additionally, we found histology type was significantly associated with the odds of clinical trial participation. Specifically, patients with T-cell lymphoma (OR, 1.62; P = .12) and mantle cell lymphoma (OR, 2.93; P < .001) were more likely to participate in clinical trials than those with DLBCL, whereas patients with marginal zone lymphoma had lower participation rates (OR, 0.56; P =.009) than those with DLBCL. There was no significant difference in participation based on sex, race/ethnicity, ADI, or the nSES variable related to education attainment (percentage population aged >25 years with no high school diploma). Interestingly, the observed significant association between trial participation and ADI in univariant analysis was no longer present in the covariate-adjusted logistic regression models. In these adjusted models, age appeared to show the strongest association with participation. When we adjusted the same covariates except histology, a separate model for the DLBCL subgroup showed that Hispanic patients were significantly less likely to enroll in trials (OR, 0.36; P = .001) than their NHW counterparts (Figure 1B). In the FL subgroup analysis, age demonstrated significant inverse association with the odds of clinical trial participation (estimated degrees of freedom = 2.60; P = .002) (Figure 1C). Regarding noninterventional trials and interventional nontherapeutic trials, the analysis revealed no significant associations across any demographic, nSES variables, ADI, or travel distance. The geospatial distribution of patients’ residencies by trial participation is shown in Figure 1.
Discussion
It is estimated that only 2% to 3% of adult patients with cancer participate in clinical trials.10,30-34 Participation rates differ significantly by care setting, with much higher participation rates at academic sites (∼16%) than community sites (∼7%).34 It is also important to note that 85% of patients receive treatment in the community, compared with only ∼15% in larger academic centers.34 Barriers to clinical trial recruitment vary by institutional setting, and a large meta-analysis found that 56% of patients with cancer lacked local trial availability, a significant structural barrier.34 This study also found that structural factors such as trial availability and clinical factors (including eligibility criteria) together precluded 77.1% of patients from having the option to participate in trials.34 The underrepresentation of minority patients has been reported to be even more pronounced in hematologic malignancies, with 1 meta-analysis showing that Black patients represented 1.5% of participants in the phase 3 DLBCL trials examined.33 Our analysis explored clinical trial enrollment patterns among patients with lymphoma at a single high-volume institution and examined the association with race, ethnicity, and neighborhood socioeconomic variables. Participation rates in our cohort were quite high across NHL subtypes, with an overall clinical trial participation rate of 30% and 21% in interventional therapeutic clinical trials. Although clinical trial participants in our cohort were predominantly NHW and male, the racial composition of our cohort’s trial participants largely reflected the patient population treated at the institution. The only significant difference detected was among patients with DLBCL, among whom Hispanic patients had 63% lower odds of enrolling in trials than their NHW counterparts.
Although we identified associations between various nSES variables and clinical trial participation, the most significant associations were with age and histology. Interestingly, enrollment in clinical trials did not vary significantly by individual race or neighborhood racial composition, which stands in contrast to previously described lower participation rates among these groups in other studies.31,35-38 It is important to note that although clinical trial participation at this single center was representative of the patient population at the institution, this patient population does not entirely reflect the broader population of patients with NHL in the local community. There are many potential reasons for the observed differences that may provide some insight into the differences seen in representativeness in clinical trial populations. Firstly, MDACC attracts patients from all across the globe, with ∼43% of the new patients with lymphoma residing outside of Texas and 4% being international patients. It is important to note that patients who can travel to receive care are likely to have more resources and less likely to live in neighborhoods with the sociodemographic characteristics that we identified to be associated with lower clinical trial participation.
Another notable feature of our study population was that 93% of patients had private insurance or Medicare insurance, which is markedly different from the local community. According to the US Census Bureau data, Texas leads the country in the number of uninsured residents (18%).39 Moreover, in 2020 in Harris County, 20.4% of all residents were uninsured, including 26.9% of individuals between the ages of 19 to 64 who were without insurance.40,41 Medicaid accounted for 15.3% of insurance coverage in Harris County, but only 1.2% of our study population had Medicaid as their primary insurance.41 Other hospital systems within Harris County provide care for patients with other insurance types such as Harris Health that provides care for indigent, uninsured, Medicaid, and underinsured patients. Expanding access to clinical trials for patients with lymphoma with these insurance types will require dedicated efforts to increase the quantity and variety of clinical trials in locations where these patients receive care.
A few unique features about our environment include the patient population, the staff, available resources, and the institution itself. It is important to note that The Department of Lymphoma and Myeloma at MDACC is 1 of the largest multidisciplinary programs with 34 research and clinical faculty and >250 department members and trainees. It houses a robust portfolio of lymphoma clinical trials with dozens of active therapeutic trials at any given time and dedicated programs to support clinical trial enrollment such as specialized research nurse coordinator, patient navigators, and financial assistance programs. Additionally, many patients are drawn to the institution specifically for novel therapies, often with specific trials in mind, which results in a patient population that is inherently more inclined to participate in clinical trials. Additionally, there are specialized departments such as the behavioral science and cancer health disparities, which champions several programs aimed at improving diversity of clinical trial participants and surmounting numerous obstacles that might impede access. The institution invests in resources such as a learning center site, which provides information about clinical trials through various media, including education reading material and informative videos to provide multimedia modes of information dissemination. There are also programs matching patients with similar diagnoses to facilitate peer support groups, which can help patients learn from the experiences of other patients, including those previously participated in trials. Lastly, there have been significant strategic investments in programs such as the growing patient navigator program that provides trained research navigators to help patients understand clinical trial process and overcome barriers to participation including some financial challenges.
Despite its strengths, this study has other limitations. Due to the retrospective nature of our study, we were unable to control for all possible confounders including selection bias arising from our eligibility criteria. nSES may not serve as a surrogate for individual-level SES (iSES), which measures different dimensions of social determinants of health that can also affect clinical trial participation and outcomes. Although nSES provides insight about the communities that patients come from, discrepancies between iSES and nSES and complex interactions between the 2 are not well accounted for in our study design. These measures have been shown useful when evaluated jointly and are not mutually exclusive.42-44 The patient address and insurance status at the time of diagnosis are used as static measures but may change over the course of a patients’ care, which was not captured in our analysis. Although we focused on primary insurance, it is important to note that many patients may have multiple insurance providers. For example, patients aged ≥65 years may have both Medicare and other supplementary insurance plans including Medicaid dual enrollment or private insurance. Our results may not be generalizable to the US population because the highly selected patient population seen at MDACC differs from patients receiving care in other health care settings. However, these data do indicate that in clinical environments with strong infrastructure and a focus on clinical trials, similar clinical trial participation across racial and ethnic groups can be achieved.
It is important to note that the current categorization of race and ethnicities and how we collect these data are intrinsically flawed. Although self-reported race and ethnicity are generally regarded as more reliable than chart-abstracted groupings, current categories are too simplistic. Race is a social construct that groups people of common ancestry by means of physical characteristics, such as hair type, color of eyes and skin, and stature. However, these groupings, based on limited biologic basis, can have significant social consequences. Ethnicity, on the other hand, refers to a large group of people with a shared culture, language, history, set of traditions, and other features. These 2 concepts, although often reported together, represent very different concepts and simplify the categorization of diverse and heterogenous populations. For example, the current category of Asian (non-Hispanic Asian) inadequately captures a very diverse groups of ethnicities from various regions of a continent, including people of Japanese, Chinese, Indian, and Vietnamese heritage, to name a few, with different languages and cultures. Analogous inaccuracies occur for other groups. It is important to interpret the results of our findings in this context.
Much of the literature about accrual to clinical trials has focused on the patient’s willingness to participate. Prospective attitude surveys and recent meta-analysis studies show high level of willingness to participate in clinical trials when offered the opportunity, with more than half of patients agreeing with no difference in acceptance rates by race.45-47 Future efforts to improve lymphoma trial participation should shift from solely focusing on patient willingness to other factors. For example, programs such as the incorporation of oncology nurse navigation have been shown to be an efficacious and cost-effective strategy for increasing clinical participation among minority patients with cancer.48
To improve enrollment rates across diverse settings, it is important to evaluate the effectiveness of potential interventions such as financial assistance based on income level/poverty status, housing support, clinical trial educational resources, and additional counseling services. Moreover, proactively screening patients based on their address may identify patients from socioeconomically disadvantaged neighborhoods who may benefit from these additional services. Prospectively evaluating the relationships between individual SES and nSES and clinical trial participation also will be important in developing future interventions.
Academic centers with robust trial portfolios still face challenges with recruiting participants. Several studies have shown that stringent eligibility criteria in lymphoma clinical trials such as criteria for stage, organ function, HIV status, history of other malignancy, self-reported comorbidity burden, and other laboratory cut offs may unnecessarily exclude potentially eligible patients without a significant impact on trial outcomes.49-51 These criteria may preferentially exclude patients with high-risk disease in need of urgent treatment, older patients, or minority patients who might have a higher burden of other cancers and comorbidities.52 Modifications to eligibility criteria to balance between safety and access could significantly expand trial accessibility, facilitate enrollment of a clinically diverse study population, and ensure that patients are not unnecessarily excluded from trial participation.50 Recommendations to modernize eligibility criteria for cancer clinical trials have been suggested in previous publications.53,54 Accessibility to trial sites is another critical factor. Barriers to access comprehensive cancer centers for low-income patients can be reduced by expanding coverage and clinical trials availability at community cancer centers where many receive care or implementing decentralized clinical trials. Trial sponsors should develop policies to expand their selection of sites, taking into consideration the diversity of patients treated across sites.
For future prospective trials, it will be critical to capture data on social determinants of health to better assess how well study populations reflect the demographics affected by a particular disease. Relying solely on race, ethnicity, and currently collected demographic data may not fully capture the socioeconomic variation in clinical trial participation and neglect important factors contributing to disparities. These data will be crucial in informing future efforts and trial design considerations to ensure meaningful representation in trials. Additionally, incorporating pharmacogenomics data in future prospective trials will provide valuable insights into the efficacy of novel therapies among patients of different ancestries, offering a more nuanced understanding than the often biologically limited categories of race and ethnicity.
There are numerous strategies used at MDACC to improve access to clinical trials. These include systematic screening of new and return patients for trials, offering a clinical study to all new patients, the broad availability of observational and biomarker studies, and the use of patient navigation for clinical trials and clinical trial nurse coordinators. It is also notable that physicians at this site have trial leadership roles as principal investigators and are active in recruiting patients to clinical trials. This study shows that it is possible to enroll patients across all racial/ethnic groups at rates similar to the demographics of the overall patient population and provides proof of principle for using these methods. It will be important to prospectively examine the efficacy of the previously mentioned strategies in improving enrollment rates of all patients and minority patients to lymphoma clinical trials and to extrapolate findings from these studies to other contexts.
iSES and nSES have been identified as independent and joint determinants of cancer disparities.3-9 In this large single-center academic cohort, multivariable models identified age and diagnosis as independent predictors of clinical trial participation, whereas race, sex, travel distance, and nSES were not significant factors. Additional challenges faced by potential cancer clinical trial participants such as frequent office visits and financial concerns are frequently cited as limiting factors, particularly for vulnerable populations. Recognizing this deficiency, the FDA offers guidance to help achieve proportional representation on clinical trials including evaluation for and elimination of restrictive eligibility criteria, increasing community engagement, and decreasing financial, travel, and other burdens on participants that could be barriers to participation.55,56 The National Academies of Science, Engineering and Medicine 2022 report provides 17 system-level recommendations with complete policy assessments to improve the representation of underrepresented and excluded populations in clinical trials and achieve lasting change.57 These include increasing accessibility and transparency of data on trial participation and recruitment efforts, journals and publishers requiring information on the representativeness of trials and studies for submissions, increasing federal incentives to institutions and pharmaceutical companies for increasing diversity in trials, ensuring that trials provide adequate compensation for research participants, increasing the diversity of the research workforce and leadership, and investing in community research infrastructure as well as strategic partnerships.
Our study examined an academic institution with policies, procedures, and a predominantly insured patient population. In this setting, we identified age as the predominant factor associated with decreased participation in lymphoma clinical trials. Examination of multicenter data sets including community practices in which underinsured patients received care and evaluation of interventions to improve access to clinical trials are needed to fully understand and address the broader spectrum of barriers.
Acknowledgments
This work was supported in part by a research grant agreement with the Leukemia and Lymphoma Society IMPACT program, MorphoSys US Inc (PI: C.R.F.); the Cancer Prevention and Research Institute of Texas (RR190079), where C.R.F. is a CPRIT Scholar in Cancer Research; and the National Cancer Institute of the National Institutes of Health under award number K24CA208132. This work was supported in part by a research funding from Conquer Cancer Foundation of ASCO Young Investigator Award and the American Society of Hematology Minority Hematology Fellow Awards received by C.N. Additionally, this work was also supported by the Center for Clinical and Translational Research, an MD Anderson resource.
Any opinions, findings, and conclusions expressed in this material are those of the authors and do not necessarily represent the official views of the National Institutes of Health, American Society of Clinical Oncology, Conquer Cancer, or the American Society of Hematology.
Authorship
Contribution: C.N. and C.R.F. conceptualized and designed the study; all authors were responsible for data acquisition; C.N., C.R.A., A.A.A., C.X.B., and C.R.F. analyzed the data; C.N., C.R.A., L.J.N., and C.R.F. interpreted the data; C.N., C.R.A., A.A.A., L.J.N., and C.R.F. drafted the manuscript; and all authors critically reviewed and revised the manuscript, have approved the manuscript as submitted, and agreed to the published version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Chijioke Nze, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77006; email: ccnze@mdanderson.org.
References
Author notes
Data can be shared with those who request it. Data will be shared upon reasonable request to the corresponding author, Chijioke Nze (ccnze@mdanderson.org).
The full-text version of this article contains a data supplement.