Key Points
HRQoL improved in all 6 patients with APDS receiving selective PI3Kδ inhibitor leniolisib for up to 6 years, with minimal toxicities.
Patients with APDS receiving leniolisib saw beneficial changes in the mean percentage of lymphocyte subsets and clinical manifestations.
Visual Abstract
Activated phosphoinositide 3-kinase delta (PI3Kδ) syndrome (APDS) is an inborn error of immunity that manifests as immune deficiency and dysregulation; symptoms include frequent infections and lymphoproliferation. In our dose-finding and phase 3 placebo-controlled trials, treatment with the selective PI3Kδ inhibitor leniolisib reduced lymphoproliferation and normalized lymphocyte subsets. Here, we present 6 years of follow-up from the 6 adult patients in the original dose-finding trial receiving leniolisib. We used data from the ongoing open-label extension study, which was supplemented at later time points by investigators, including health-related quality of life (HRQoL) assessed through a clinician-reported questionnaire. We observed improvements in HRQoL: 5 of 6 patients experienced an increase in physical capabilities and socialization, and a decrease in prescribed medications. Immune subsets improved in all patients: mean transitional B-cell levels decreased from 38.17% to 2.47% and the CD4:CD8 T-cell ratio normalized to 1.11. Manifestations seen before and within the first year of leniolisib exposure, such as infections and gastrointestinal conditions, attenuated after year 2, with few new conditions emerging out to year 6. Thrombocytopenia or lymphopenia remained present in half of patients at year 6. Of 83 adverse events through year 5, 90.36% were grade 1; none were grade 4/5 nor deemed leniolisib related. Collectively, we saw an enhancement in HRQoL as well as durable changes in lymphocyte subsets and clinical manifestations, further supporting the use of leniolisib as a long-term therapeutic option for the treatment of APDS. This trial was registered at www.ClinicalTrials.gov as #NCT02859727.
Introduction
Activated phosphoinositide 3-kinase delta (PI3Kδ) syndrome (APDS) is an inborn error of immunity (IEI) driven by hyperactive PI3Kδ signaling resulting from pathogenic heterozygous variants in PIK3CD or PIK3R1 that encode the PI3Kδ heterodimer.1-3 PI3Kδ hyperactivation alters lymphocyte development and function, causing immune deficiency and dysregulation.4-6
B- and T-cell alterations cause recurrent sinopulmonary infections, herpesvirus viremia, chronic lymphoproliferation including nodular lymphoid hyperplasia of the gastrointestinal and respiratory tracts, and autoimmunity.5,7-9 Patients have a high risk of developing lymphoma, which may occur, in part, because of uncontrolled antigen- or Epstein Barr virus (EBV)-driven PI3Kδ hyperactivity in B cells and impaired T-cell–mediated tumor surveillance.4,5,8-11
Chronic conditions such as recurrent infections and bronchiectasis seen in APDS may negatively affect perceived health.8,12 Children with chronic illness were reported to have more illness-related school absenteeism than healthy peers, affecting academic performance and social well-being.13-15 Similarly, chronic illness in adults was a barrier to employment, increased the number of missed workdays, and was associated with lower health-related quality of life (HRQoL) scores.16,17
HRQoL can also be affected by treatment burden, a lifelong possibility for patients with IEIs.18,19 Current treatment strategies for APDS are empirical and include prophylactic antimicrobials, immunoglobulin replacement therapy (IRT), immunomodulatory therapies, surgeries, and hematopoietic stem cell transplantation. Most do not target the root cause of disease: hyperactive PI3Kδ signaling.4,5,8 Selective PI3Kδ inhibition addresses disease pathogenesis.3 We previously reported reduced PI3Kδ/AKT pathway hyperactivity and ameliorated immune dysregulation and deficiency in 2 studies of patients with APDS treated with PI3Kδ inhibitor leniolisib.20,21 In our recent long-term open-label extension (OLE) study, leniolisib was well tolerated, with safety and efficacy outcomes remaining durable up to years 5 and year 1, respectively.22
Here, we expand upon efficacy outcomes not addressed in the OLE and present case vignettes of 6 adult patients with APDS who received leniolisib for 6 years. Given the paucity of validated HRQoL instruments for IEIs, we developed a standardized clinician-reported questionnaire to elucidate APDS-specific aspects of HRQoL, including clinical signs, physical abilities, socialization, schooling/employment, healthcare resource use, and general health status before and after receiving leniolisib.23,24 To our knowledge, this work represents 1 of the longest targeted treatment studies in the IEI field.
Methods
Patients and treatment
Patients were enrolled in a dose-finding trial (DFT) and are participating in an ongoing extension study (ClinicalTrials.gov identifier: NCT02859727) examining the long-term use of leniolisib in patients with APDS.20,22 Full eligibility criteria were described previously.20,22 The 6 patients, who are from the United States (n = 3), Canada (n = 1), Czech Republic (n = 1), and the Netherlands (n = 1), receive 70 mg leniolisib orally every 12 hours in the OLE (supplemental Figure 1).
Clinical and laboratory outcomes
The OLE’s primary end point is safety, including adverse events (AEs), laboratory parameters, and physical examinations.22 Safety measurements from the clinical study report (CSR) were collected out to the latest time point with available data (generally year 6; data cutoff in December 2022); efficacy measurements to approximately year 1. AEs, viral load of EBV and cytomegalovirus measured by polymerase chain reaction, and lymphoproliferation data for the spleen, liver, and lymph nodes are reported from the CSR; details regarding lymphoproliferation measurement were reported previously.21,22
Cytopenias were assessed via safety data reported in the CSR, with some data at year 6 being provided by trial investigators (medical doctors and registered nurses) via a standardized file (supplemental Appendix 1). Immunoglobulin levels and lymphocyte subsets were reported out to year 1 by the CSR; afterward by investigators. Immunoglobulin G (IgG) levels at DFT baseline were also provided by investigators. Infections, organ function (eg, gastrointestinal, neurological, dermatologic, and respiratory) and IRT use were collected via the CSR; year 6 via investigators. No statistical analyses were performed.
Because of coronavirus disease 2019 (COVID-19) restrictions, complete lymphocyte phenotype data at year 5 were only available for patient 3 (P3). The only data past year 1 for P5 were total CD4+ and CD8+ T cells. Markers for immune subsets differed between patients (supplemental Table 1).
HRQoL and case vignettes
HRQoL was assessed out to year 5 of treatment via patient answers to the Short-Form Health Survey 36, Work Productivity and Activity Impairment plus Classroom Impairment Questionnaire, and patient and physician global assessments. To expand on these results, trial investigators completed a standardized questionnaire in December 2022 and responded to queries to clarify responses (supplemental Appendix 2). The questionnaire included open-ended and “yes/no” objective and subjective questions that addressed patient symptoms, response to COVID-19 infections and vaccine challenges, physical abilities, socialization, healthcare resource use, and general health before and during treatment with leniolisib. We used responses to assess HRQoL and construct case vignettes. No statistical analyses were performed.
Trial oversight
We used data from the DFT and an interim analysis of an ongoing OLE study. Novartis, in collaboration with investigators, designed both studies. Novartis oversaw trial conduct and analyzed data, with Pharming Group, NV acquiring rights to leniolisib in 2019 and full trial management in December 2022. Both studies were conducted and reported in accordance with the protocols (including the statistical analysis plan). Data were gathered locally, and protocol-defined laboratory samples and imaging were processed centrally. The trials were conducted in accordance with the Declaration of Helsinki and the International Council for Harmonization Good Clinical Practice guidelines. Independent ethics committees or institutional review boards at each center approved the protocols. Patients and/or their guardians provided written informed consent/ascent. An independent data and safety monitoring committee monitored safety and protocol compliance.
Independent ethics committees or institutional review boards at each center approved the protocols.
Results
Patients
This study included 4 male and 2 female patients currently aged 23 to 39 years with a gain-of-function variant in PIK3CD along with clinical symptoms compatible with APDS (Table 1). Aggregate data are described in text, whereas individual patient data are reported in case vignettes and tables.
HRQoL
Patient- and physician-reported outcomes
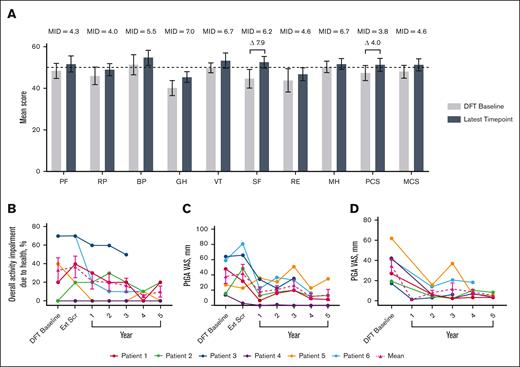
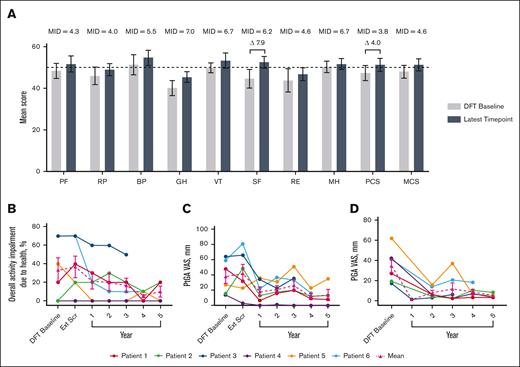
Short-Form Health Survey 36 mean physical component score increased by 4.0 points and social functioning by 7.9 at year 5, both of which are considered clinically meaningful improvements (Figure 1).25 Mean overall activity impairment because of health, as assessed via the Work Productivity and Activity Impairment plus Classroom Impairment Questionnaire, reduced by 70.0%. Patient and physician general assessments showed reductions in mean disease activity of 63.2% and 86.3%, respectively.
Changes in HRQoL over time. (A) Normed subscale and component scores from the SF-36 survey, with higher scores indicating greater HRQoL.26 MIDs for patients with IEI are from the literature.25 The latest time point for P1, P2, P3, P4, P5, and P6 are as follows: year 5, 5, 3, 5, 5, and 4, respectively. Differences in scores between DFT baseline and latest time point that exceed MIDs are illustrated with a delta symbol (Δ) followed by the value of the difference. Dotted line indicates general US population average. (B) Individual and mean WPAI-CIQ overall activity impairment because of health scores over time, with lower scores indicating less impairment because of health. n values for DFT baseline, Ext Scr and years 1, 2, 3, 4, and 5 are as follows: 6, 6, 6, 6, 6, 5, and 4, respectively. (C) Individual and mean patient general assessment scores are shown with lower scores indicating a reduction in disease activity. n values for DFT baseline, Ext Scr and years 1, 2, 3, 4, and 5 are as follows: 6, 6, 6, 6, 6, 5, and 4, respectively. (D) Individual and mean physician general assessment scores are shown with lower scores indicating a reduction in disease activity. There were no values reported for Ext Scr. n values for DFT baseline and years 1, 2, 3, 4, and 5 are as follows: 6, 1, 6, 6, 5, and 4, respectively. Error bars are standard error of the mean. BP, bodily pain; Ext Scr, extension screening; GH, general health; MCS, mental component score; MH, mental health; MID, minimally important difference; PCS, physical component score; PF, physical functioning; PGA, physician general assessment; PtGA, patient general assessment; RE, role (emotional); RP, role (physical); SF-36, Short-Form Health Survey 36; SF, social functioning; VT, vitality; WPAI-CIQ, Work Productivity and Activity Impairment Questionnaire plus Classroom Impairment Questionnaire.
Changes in HRQoL over time. (A) Normed subscale and component scores from the SF-36 survey, with higher scores indicating greater HRQoL.26 MIDs for patients with IEI are from the literature.25 The latest time point for P1, P2, P3, P4, P5, and P6 are as follows: year 5, 5, 3, 5, 5, and 4, respectively. Differences in scores between DFT baseline and latest time point that exceed MIDs are illustrated with a delta symbol (Δ) followed by the value of the difference. Dotted line indicates general US population average. (B) Individual and mean WPAI-CIQ overall activity impairment because of health scores over time, with lower scores indicating less impairment because of health. n values for DFT baseline, Ext Scr and years 1, 2, 3, 4, and 5 are as follows: 6, 6, 6, 6, 6, 5, and 4, respectively. (C) Individual and mean patient general assessment scores are shown with lower scores indicating a reduction in disease activity. n values for DFT baseline, Ext Scr and years 1, 2, 3, 4, and 5 are as follows: 6, 6, 6, 6, 6, 5, and 4, respectively. (D) Individual and mean physician general assessment scores are shown with lower scores indicating a reduction in disease activity. There were no values reported for Ext Scr. n values for DFT baseline and years 1, 2, 3, 4, and 5 are as follows: 6, 1, 6, 6, 5, and 4, respectively. Error bars are standard error of the mean. BP, bodily pain; Ext Scr, extension screening; GH, general health; MCS, mental component score; MH, mental health; MID, minimally important difference; PCS, physical component score; PF, physical functioning; PGA, physician general assessment; PtGA, patient general assessment; RE, role (emotional); RP, role (physical); SF-36, Short-Form Health Survey 36; SF, social functioning; VT, vitality; WPAI-CIQ, Work Productivity and Activity Impairment Questionnaire plus Classroom Impairment Questionnaire.
Infections and treatment burden
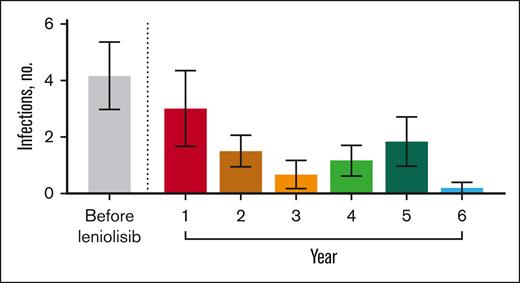
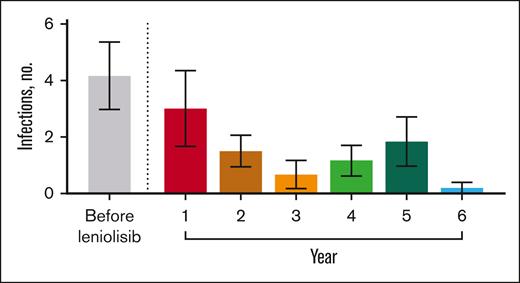
Before receiving leniolisib, all patients had difficulty fighting infections (Table 3). While receiving leniolisib, the number or severity of infections decreased for all, from a mean of 3 in year 1 to 0.2 in year 6 (Figure 2).
Number of infections over time. Mean number of infections before and throughout exposure to leniolisib. n values for before leniolisib and years 1, 2, 3, 4, 5, and 6 are as follows: 6, 6, 6, 6, 6, 6, and 5, respectively. “Before leniolisib” comprises multiple years of recurrent/unresolved infection history. Post-leniolisib exposure includes only acute infections. Dotted line separates infections that occurred before and during treatment with leniolisib. Error bars are ± standard error of the mean.
Number of infections over time. Mean number of infections before and throughout exposure to leniolisib. n values for before leniolisib and years 1, 2, 3, 4, 5, and 6 are as follows: 6, 6, 6, 6, 6, 6, and 5, respectively. “Before leniolisib” comprises multiple years of recurrent/unresolved infection history. Post-leniolisib exposure includes only acute infections. Dotted line separates infections that occurred before and during treatment with leniolisib. Error bars are ± standard error of the mean.
Clinician visits per year and number of specialists seen reduced for all. During the course of the study, patients received an average of 13.3 (range, 5-22) prescription medications excluding leniolisib; at year 6, 3.83 (range, 2-7). Largely, prophylactic or therapeutic antibiotic use decreased. Two of 5 patients discontinued IRT. Changes in medication were not protocolized and occurred at investigator discretion (supplemental Table 2).
Physical capabilities
All patients had been sedentary or physically limited because of shortness of breath or fatigue. Physical activity improved in 5 of 6 patients within 6 months of receiving leniolisib. Four patients were able to walk several miles, run, or partake in physically demanding activities.
Fatigue
Fatigue affected the ability to attend school, work, or complete daily chores in 4 of 6 patients. During treatment, patients experienced reduced fatigue severity, increased energy, and ability to pursue activities.
Mental well-being
Two of 6 patients experienced anxiety before receiving leniolisib. One case resolved during year 1 of treatment. Questionnaires reported increased socialization and the development of a more positive outlook on life, and mood improved in 4 of 6.
Socialization
Social life improved in 5 of 6 patients within 1 year of treatment. Two of 5 returned to school and created friendships. Activities included traveling overseas to visit friends and increasing socialization. Half used online resources such as video games for socializing during the COVID-19 pandemic.
School and work
At enrollment, half of patients missed school or required homeschooling. The same 3 patients finished high school, college, or secondary education during the trial. Five of 6 joined the workforce via remote or in-person jobs. One patient finished high school before the trial and did not seek higher education or employment.
Clinical and laboratory outcomes
Means correspond to all patients with data at reported time point.
Lymphoproliferation
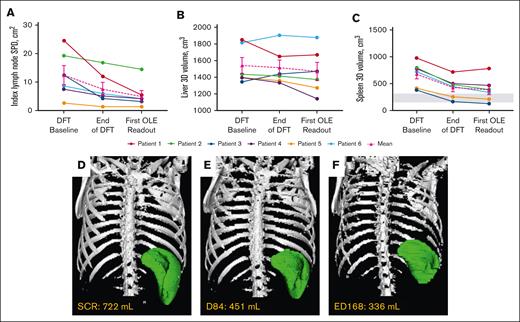
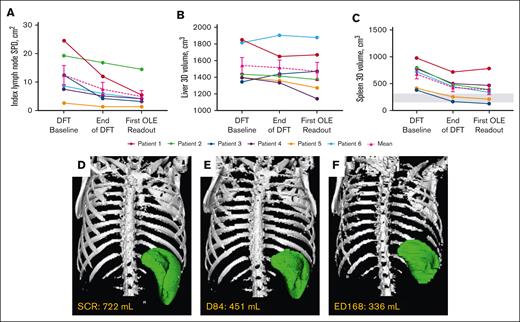
Mean index lymph node sum of product of diameters decreased 56.7%, liver volume by 4.7%, and spleen volume by 43.1% around year 1 (Table 2; Figure 3). Collectively, lymphadenopathy and splenomegaly decreased in all patients, with spleen size reaching normal range in 2 of 6 patients. Hepatomegaly was absent in 5 of 6 patients through year 6, and persistently mild in 1 patient.
Changes in lymphoproliferation parameters. (A) Individual and mean untransformed SPD of index lymph nodes. n values for all time points are 6. Reference range (≤1.5 × 1.5 cm) not shown because up to 6 lymph nodes may be counted per patient.27 (B) Individual and mean liver volumes. n values for all time points are 6. (C) Individual and mean spleen volumes. n values for all time points are 6. Gray box indicates the reference range for adults.28 Imaging used to determine lymphoproliferation for each patient at their first OLE readout was completed around year 1 (either ED168 or 252) of the OLE study. (D-F) Representative radiographic renderings of spleen volume from P6 at (D) screen of DFT (SCR), (E) end of the DFT (D84), and (F) first extension study readout (ED168) in which splenomegaly was absent. P6 had a 2-month gap in treatment between the end of part 1 and entry into the extension. D, day; ED, extension day; SCR, screen.
Changes in lymphoproliferation parameters. (A) Individual and mean untransformed SPD of index lymph nodes. n values for all time points are 6. Reference range (≤1.5 × 1.5 cm) not shown because up to 6 lymph nodes may be counted per patient.27 (B) Individual and mean liver volumes. n values for all time points are 6. (C) Individual and mean spleen volumes. n values for all time points are 6. Gray box indicates the reference range for adults.28 Imaging used to determine lymphoproliferation for each patient at their first OLE readout was completed around year 1 (either ED168 or 252) of the OLE study. (D-F) Representative radiographic renderings of spleen volume from P6 at (D) screen of DFT (SCR), (E) end of the DFT (D84), and (F) first extension study readout (ED168) in which splenomegaly was absent. P6 had a 2-month gap in treatment between the end of part 1 and entry into the extension. D, day; ED, extension day; SCR, screen.
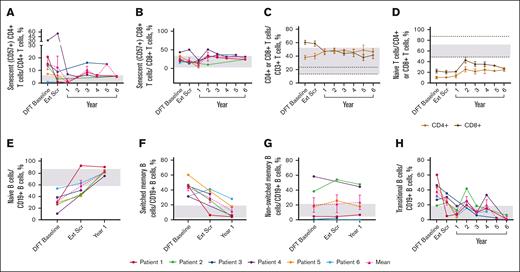
Immune profiling
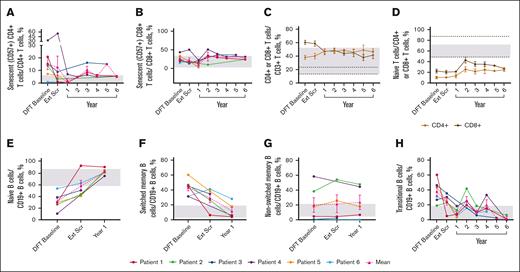
The mean percentage of senescent (CD57+) CD4+ T cells (2.0%) reached normal range by year 1 and generally remained within normal limits (WNLs) through year 6; senescent CD8+ T cells remained WNLs (Figure 4A-B). The mean percentage of total CD8+ T cells reduced to 41.77%, and total CD4+ T cells increased to 46.39%, normalizing the CD4:CD8 T-cell ratio of 0.63 at the DFT baseline to 1.11 at year 6 (Figure 4C). Neither naïve CD4+ nor CD8+ T cells reached normal limits (Figure 4D). Mean percentage of effector memory T cells remained unchanged, whereas terminally differentiated effector memory CD4+ and CD8+ T cells remained WNLs until year 6 (supplemental Figure 2A-B). CD8+ central memory T cells remained WNLs throughout the study; CD4+ central memory T cells approached normal range by year 6 (48.40%; supplemental Figure 2C).
Sustained changes in B- and T-cell subsets. (A) Individual and mean senescent (CD57+) CD4+ T-cell percentages over time. n values for part 1 baseline, Ext Scr, and years 1, 2, 3, 4, 5, and 6 are as follows: 6, 6, 4, 1, 3, 2, 1, and 3, respectively. (B) Individual and mean senescent CD8+ T-cell percentages over time. n values for part 1 baseline, Ext Scr, and years 1, 2, 3, 4, 5, and 6 are as follows: 6, 6, 4, 3, 3, 2, 1, and 3, respectively. (C) Mean CD4+ and CD8+ T-cell percentages overtime. n values for part 1 baseline, Ext Scr, and years 1, 2, 3, 4, 5, and 6 are as follows: 6, 6, 6, 5, 6, 5, 3, and 4, respectively. (D) Mean naïve T-cell percentages (CD4+ or CD8+ CD45RACD62L+) over time. n values for part 1 baseline, Ext Scr, and years 1, 2, 3, 4, 5, and 6 are as follows: 6, 6, 4, 4, 5, 2, 1, and 3, respectively. (E) Individual and mean naïve B-cell percentages (CD19+CD27−CD10−) over time. n values for DFT baseline, Ext Scr, and year 1 are as follows: 6, 5, 5. (F) Individual and mean switched memory B-cell percentages (CD19+CD27+IgD−) over time. n values for DFT baseline, Ext Scr, and year 1 are as follows: 6, 5, and 5, respectively. (G) Individual and mean nonswitched memory B-cell percentages (CD19+CD27+IgD+) over time. n values for DFT baseline, Ext Scr, and year 1 are as follows: 6, 4, and 5, respectively. (H) Individual and mean transitional B-cell (CD19+CD27−CD10+ or CD20+CD10+) percentages over time. n for DFT baseline, Ext Scr, and years 1, 2, 3, 4, 5, and 6 are as follows: 6, 6, 5, 4, 5, 3, 1, and 3, respectively. Normal ranges are from personal communication (panels A-B; Manish Butte, University of California, Los Angeles, email, 29 March 2022) and literature (panels C-H).29,30 In panels A-B,E-H, gray boxes indicate normal ranges. In panels C-D, gray boxes indicate normal ranges for CD4+ cells, and dotted lines indicate normal ranges for CD8+ cells. Error bars are ± standard error of the mean. Ext Scr, extension screening.
Sustained changes in B- and T-cell subsets. (A) Individual and mean senescent (CD57+) CD4+ T-cell percentages over time. n values for part 1 baseline, Ext Scr, and years 1, 2, 3, 4, 5, and 6 are as follows: 6, 6, 4, 1, 3, 2, 1, and 3, respectively. (B) Individual and mean senescent CD8+ T-cell percentages over time. n values for part 1 baseline, Ext Scr, and years 1, 2, 3, 4, 5, and 6 are as follows: 6, 6, 4, 3, 3, 2, 1, and 3, respectively. (C) Mean CD4+ and CD8+ T-cell percentages overtime. n values for part 1 baseline, Ext Scr, and years 1, 2, 3, 4, 5, and 6 are as follows: 6, 6, 6, 5, 6, 5, 3, and 4, respectively. (D) Mean naïve T-cell percentages (CD4+ or CD8+ CD45RACD62L+) over time. n values for part 1 baseline, Ext Scr, and years 1, 2, 3, 4, 5, and 6 are as follows: 6, 6, 4, 4, 5, 2, 1, and 3, respectively. (E) Individual and mean naïve B-cell percentages (CD19+CD27−CD10−) over time. n values for DFT baseline, Ext Scr, and year 1 are as follows: 6, 5, 5. (F) Individual and mean switched memory B-cell percentages (CD19+CD27+IgD−) over time. n values for DFT baseline, Ext Scr, and year 1 are as follows: 6, 5, and 5, respectively. (G) Individual and mean nonswitched memory B-cell percentages (CD19+CD27+IgD+) over time. n values for DFT baseline, Ext Scr, and year 1 are as follows: 6, 4, and 5, respectively. (H) Individual and mean transitional B-cell (CD19+CD27−CD10+ or CD20+CD10+) percentages over time. n for DFT baseline, Ext Scr, and years 1, 2, 3, 4, 5, and 6 are as follows: 6, 6, 5, 4, 5, 3, 1, and 3, respectively. Normal ranges are from personal communication (panels A-B; Manish Butte, University of California, Los Angeles, email, 29 March 2022) and literature (panels C-H).29,30 In panels A-B,E-H, gray boxes indicate normal ranges. In panels C-D, gray boxes indicate normal ranges for CD4+ cells, and dotted lines indicate normal ranges for CD8+ cells. Error bars are ± standard error of the mean. Ext Scr, extension screening.
The mean percentage of naïve B cells reached 58.16% at extension screening and remained WNLs through the latest time point, year 1 (82.56%; Figure 4E). Switched memory B cells fell WNLs (12.06%) at year 1; nonswitched memory B cells remained unchanged (Figure 4F-G). Elevated transitional B cells decreased to 2.47% and largely remained WNLs through year 6 (Figure 4H).
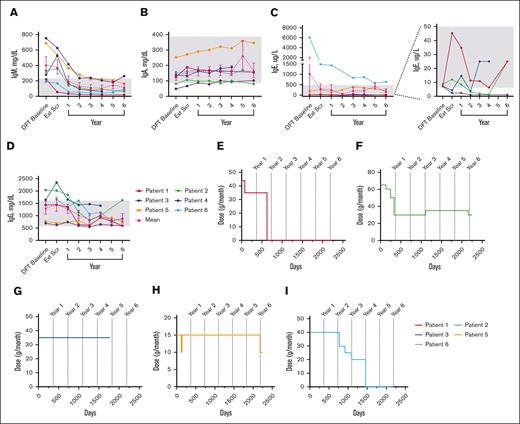
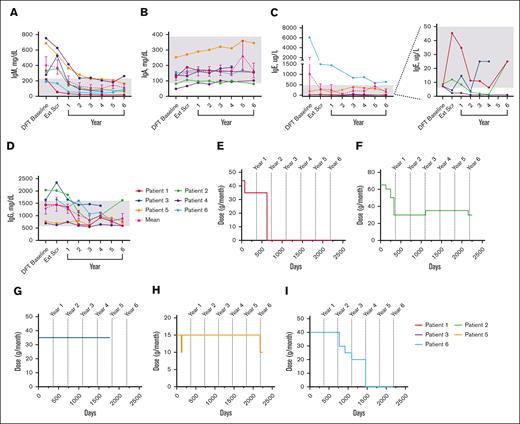
Mean IgM levels fell WNLs by year 1 (198.5 mg/dL); IgA (154.3 mg/dL), IgE (306.5 ug/L), and IgG (1469.0 mg/dL) levels fell WNLs by extension screening (Figure 5A-D). IgG subclass data beyond year 1 were only available for patients 5 and 6 (see supplemental Figure 3).
Changes in serum immunoglobulin levels and IRT throughout treatment with leniolisib. (A) Individual and mean IgM values over time. n values for part 1 baseline, Ext Scr, and years 1, 2, 3, 4, 5, and 6 are as follows: 6, 6, 6, 6, 6, 6, 3, and 5, respectively. (B) Individual and mean IgA values over time. n values for part 1 baseline, Ext Scr, and years 1, 2, 3, 4, 5, and 6 are as follows: 6, 6, 6, 6, 6, 6, 2, and 5, respectively. (C) Individual and mean IgE values over time. The graph on the far left illustrates all individual and mean IgE values over time. The far-right portion depicts individual IgE values for P1 through P4 over time as indicated by the dotted lines. n values for part 1 baseline, Ext Scr, and years 1, 2, 3, 4, 5, and 6 are as follows: 6, 6, 6, 5, 6, 6, 2, and 5, respectively. (D) Individual and mean IgG values over time. n values for part 1 baseline, Ext Scr, and years 1, 2, 3, 4, 5, and 6 are as follows: 5, 6, 6, 6, 6, 6, 3, and 5, respectively. For values that were <, the actual number was entered to graph data. IRT usage in (E) P1, (F) P2, (G) P3, (H) P5, and (I) P6 over time. Day 0 is the DFT baseline. Gray boxes in panels A-D indicate age-matched normal ranges from LabCorp.31-34 Error bars are ± standard error of the mean.
Changes in serum immunoglobulin levels and IRT throughout treatment with leniolisib. (A) Individual and mean IgM values over time. n values for part 1 baseline, Ext Scr, and years 1, 2, 3, 4, 5, and 6 are as follows: 6, 6, 6, 6, 6, 6, 3, and 5, respectively. (B) Individual and mean IgA values over time. n values for part 1 baseline, Ext Scr, and years 1, 2, 3, 4, 5, and 6 are as follows: 6, 6, 6, 6, 6, 6, 2, and 5, respectively. (C) Individual and mean IgE values over time. The graph on the far left illustrates all individual and mean IgE values over time. The far-right portion depicts individual IgE values for P1 through P4 over time as indicated by the dotted lines. n values for part 1 baseline, Ext Scr, and years 1, 2, 3, 4, 5, and 6 are as follows: 6, 6, 6, 5, 6, 6, 2, and 5, respectively. (D) Individual and mean IgG values over time. n values for part 1 baseline, Ext Scr, and years 1, 2, 3, 4, 5, and 6 are as follows: 5, 6, 6, 6, 6, 6, 3, and 5, respectively. For values that were <, the actual number was entered to graph data. IRT usage in (E) P1, (F) P2, (G) P3, (H) P5, and (I) P6 over time. Day 0 is the DFT baseline. Gray boxes in panels A-D indicate age-matched normal ranges from LabCorp.31-34 Error bars are ± standard error of the mean.
IRT use
Five patients received IRT at baseline; 2 patients discontinued and 2 reduced dose (Figure 5E-I). After IRT discontinuation, immunoglobulin levels remained WNLs through year 6 for both patients, excepting consistently high IgE in 1 patient. After IRT reduction in 1 patient, IgA was low whereas IgG was high at year 6, while another patient had low IgG3 at year 5. All 4 patients experienced a total of ≤4 infections each after termination or decrease in dosage.
Clinical manifestations
Before receiving leniolisib, all patients experienced recurrent upper respiratory tract infections. Two experienced recurrent cytomegalovirus and EBV infections. During the studies, the highest observed level of EBV was ∼2000 copies per mL, which, although clinically significant, did not result in infection.35,36 Four patients were vaccinated against COVID-19; all were positive for antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein. Half of patients tested positive for SARS-CoV-2 and experienced mild symptoms. After year 2, the number or severity of infections declined in 4 of 6 patients (Figure 2).
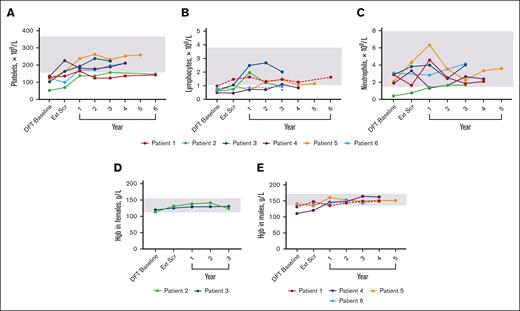
Laboratory values were used to determine the presence of cytopenias (Figure 6). Histories included thrombocytopenia (n = 6), lymphopenia (n = 4), neutropenia (n = 1), and anemia (n = 1). By the end of the DFT, 3 lymphopenia and 3 thrombocytopenia cases resolved. During continued treatment, 4 patients presented with cytopenias that included thrombocytopenia (n = 3), neutropenia (n = 1), anemia (n = 1), and lymphopenia (n = 2). At the latest time with available data, the neutropenia and anemia case resolved, whereas thrombocytopenia improved in 2 patients and resolved in 1 around year 1. Both lymphopenia cases improved but persist.
Changes in hematologic parameters over time. (A) Platelet levels for individual patients over time. n values for DFT baseline, Ext Scr, and years 1, 2, 3, 4, 5, and 6 are as follows: 6, 6, 6, 6, 6, 4, 1, and 2, respectively. There was no reported value at year 3 for P3; data from year 3.5 of treatment was used. (B) Lymphocyte levels for individual patients over time. n value for DFT baseline, Ext Scr, and years 1, 2, 3, 4, 5, and 6 are as follows: 6, 5, 6, 5, 6, 3, 1, and 1, respectively. P1 and P6 (dashed lines) were the only patients within range at the DFT. Asterisk denotes the latest time point for P6 that exceeded their individual lower limit of 0.5 × 109/L. (C) Neutrophil levels for individual patients over time. n values for DFT baseline, Ext Scr, and years 1, 2, 3, 4, and 5 are as follows: 6, 5, 6, 5, 6, 3, and 1, respectively. For lymphocytes and neutrophils, there was no reported value at year 3 or 1 for P3 and P1, respectively; data from year 3.5 and 0.5, respectively, were used. (D) Hemoglobin levels over time for individual female patients. n values for all time points are 2. There was no reported value at year 3 for P3; data from year 3.5 of treatment was used. (E) Hemoglobin levels over time for individual male patients. n values for DFT baseline, Ext Scr, and years 1, 2, 3, 4, and 5 are as follows: 4, 4, 4, 4, 4, 4, and 1, respectively. P1 and P6 (dashed lines) were the only patients within range at DFT baseline. Gray boxes indicate mean normal range that was determined by averaging lower and upper limit for all 6 patients (panels A-C), female patients only (panel D), or male patients only (panel E). Hgb, hemoglobin.
Changes in hematologic parameters over time. (A) Platelet levels for individual patients over time. n values for DFT baseline, Ext Scr, and years 1, 2, 3, 4, 5, and 6 are as follows: 6, 6, 6, 6, 6, 4, 1, and 2, respectively. There was no reported value at year 3 for P3; data from year 3.5 of treatment was used. (B) Lymphocyte levels for individual patients over time. n value for DFT baseline, Ext Scr, and years 1, 2, 3, 4, 5, and 6 are as follows: 6, 5, 6, 5, 6, 3, 1, and 1, respectively. P1 and P6 (dashed lines) were the only patients within range at the DFT. Asterisk denotes the latest time point for P6 that exceeded their individual lower limit of 0.5 × 109/L. (C) Neutrophil levels for individual patients over time. n values for DFT baseline, Ext Scr, and years 1, 2, 3, 4, and 5 are as follows: 6, 5, 6, 5, 6, 3, and 1, respectively. For lymphocytes and neutrophils, there was no reported value at year 3 or 1 for P3 and P1, respectively; data from year 3.5 and 0.5, respectively, were used. (D) Hemoglobin levels over time for individual female patients. n values for all time points are 2. There was no reported value at year 3 for P3; data from year 3.5 of treatment was used. (E) Hemoglobin levels over time for individual male patients. n values for DFT baseline, Ext Scr, and years 1, 2, 3, 4, and 5 are as follows: 4, 4, 4, 4, 4, 4, and 1, respectively. P1 and P6 (dashed lines) were the only patients within range at DFT baseline. Gray boxes indicate mean normal range that was determined by averaging lower and upper limit for all 6 patients (panels A-C), female patients only (panel D), or male patients only (panel E). Hgb, hemoglobin.
Gastrointestinal manifestations were present in 4 of 6 patients. Most emerged before or within 1 year of treatment. Reflux esophagitis, a minor case of diarrhea, and a stomach ache developed in 1 patient each in years 2 to 5.
All patients reported neurologic conditions such as anxiety and headaches in the first year; 2 patients experienced resolution.
Dermatologic symptoms were present in 4 patients before or within the first year of leniolisib exposure; no new dermatologic symptoms were reported in any patients through year 6.
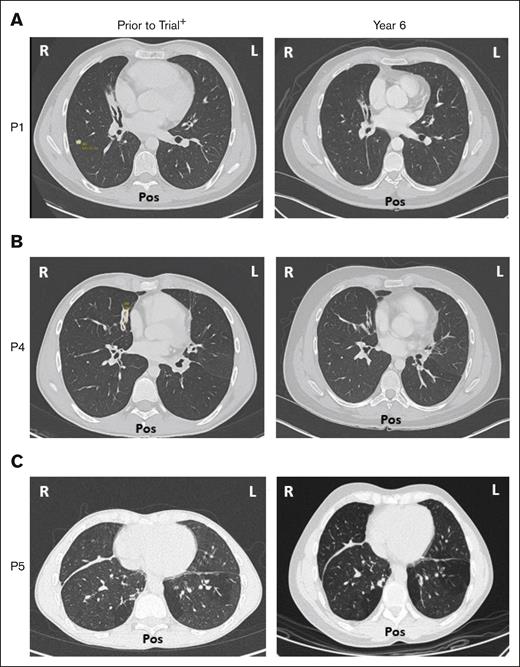
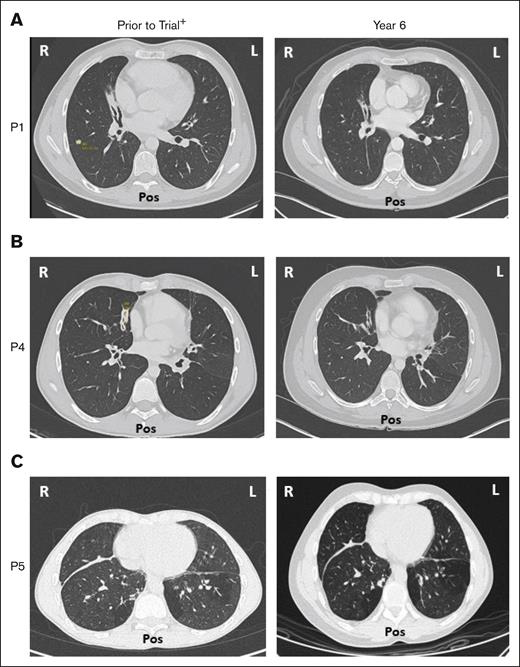
Five of 6 patients had a history of respiratory disorders. Half had bronchiectasis before receiving leniolisib; none progressed and all are stable at year 6 (Figure 7). Pulmonary function tests in 5 of 6 patients revealed no new or worsening conditions through year 6.
Bronchiectasis throughout treatment with leniolisib. Lung computed tomography scans from (A) P1, (B) P4, and (C) P5 who developed bronchiectasis before entry into the trial (left) and year 6 of treatment with leniolisib (right). L, left; Pos, posterior; R, right. +Images for P1 and P4 were taken ∼1 year before entry into the DFT, and the image for P5 was taken ∼4 years before exposure to leniolisib.
Bronchiectasis throughout treatment with leniolisib. Lung computed tomography scans from (A) P1, (B) P4, and (C) P5 who developed bronchiectasis before entry into the trial (left) and year 6 of treatment with leniolisib (right). L, left; Pos, posterior; R, right. +Images for P1 and P4 were taken ∼1 year before entry into the DFT, and the image for P5 was taken ∼4 years before exposure to leniolisib.
Three patients had a history of B-cell lymphoma and achieved full remission before receiving leniolisib. No recurrence or new lymphoma occurred in these 3 patients, nor did lymphoma appear in the other half.
Tolerability
Leniolisib was well tolerated during the DFT (Tables 5 and 6). At the 70-mg twice-a-day dose, 4 patients experienced 11 AEs; 9 were grade 1; common cold and fungal skin infection were grade 2. In the extension, 6 patients experienced 83 AEs, mostly grade 1 sinopulmonary infections in the first 2 years. Reactive arthritis in year 4 was the only serious AE. No AEs were deemed leniolisib related by investigators.
Case vignettes
Detailed medication, AE information, and immune profiling is reported in supplemental Tables 2, 5, and 6, respectively.
P1
P1 is a 24-year-old male. Before enrollment, he was frequently prescribed antibiotics and hospitalized approximately yearly for infections. He had thrombocytopenia (129 × 109/L; normal range, 140 × 109/L to 370 × 109/L) and bronchiectasis (supplemental Figure 4). P1 had difficulties attending school due to infections, gastrointestinal issues, and migraines. He experienced fatigue from IRT infusions, anxiety, and difficulty coping with treatment burden.
Throughout leniolisib treatment, P1 had 5 infections within the first 1 to 2 years: none recurrent, unlike his prior infections. P1 discontinued IRT before year 2, after which IgG levels remained WNLs and he developed 2 infections that cleared. Platelet counts increased by year 6 (144 × 109/L; normal range, 161 × 109/L to 347 × 109/L). Bronchiectasis did not progress through year 6. Over the course of the trials, he received 14 prescription medications; at year 6, 2. He experienced no hospitalizations since receiving leniolisib, and regularly visits only his immunologist.
P1’s other AEs included gastroparesis and hypertriglyceridemia. All were grade 1/2, resolved, and occurred within 2 years of treatment.
P1 graduated from high school, an online university, and works a full-time job. While finishing his last year of high school, he attended in-person schooling, a school dance, and graduated. Physical capabilities improved; he can walk and drive without difficulty, and manages his own medical care. His fatigue improved after IRT discontinuation.
P2
P2 is a 31-year-old female. Before the trial, she was fatigued and had difficulty fighting frequent sinopulmonary infections. P2 had thrombocytopenia (53 × 109/L; normal range, 140 × 109/L to 370 × 109/L) and neutropenia (0.4 × 109/L; lower limit of normal, 1.5 × 109/L). She was homeschooled because of illness and was mostly sedentary. She was diagnosed with stage IV diffuse large B-cell lymphoma at age 19 and achieved full remission before receiving leniolisib.
P2 had 2 infections during the first 2 years of treatment. Her IRT dose decreased from 65 to 30 g/mo by year 1 yet has had no infections requiring antibiotics since 2019. Thrombocytopenia improved by year 6 (148 × 109/L; normal range, 173 × 109/L to 369 × 109/L). Neutrophil counts were WNLs around year 2 and remained stable. Currently, P2 visits her local doctor regularly. Over the trials, she received 8 prescription medications; at year 6, 3.
P2’s other AEs included allergic rhinitis, dental caries, neutropenia, reactive arthritis, and elevated liver enzymes. All were grades 1/2, except grade 3 alanine aminotransferase increase and reactive arthritis; all resolved. The latter was a serious AE occurring in year 4.
Despite a learning impairment, P2 completed a post-secondary program and has a full-time job. She manages her own medical care without parental assistance, which was previously needed. She takes aerobics classes and walks for exercise. P2 experienced changes in her mood, becoming more talkative and upbeat. She traveled overseas to visit friends. P2 continues to report increases in energy with more time to pursue activities.
P3
P3 is a 23-year-old female. She experienced chronic sinopulmonary infections that required consistent antibiotics and affected her ability to attend school and socialize; she also suffered anxiety. She had lymphopenia (0.7 × 109/L; normal range, 0.8 × 109/L to 3.3 × 109/L). Her recurrent EBV infections had persistently high viral levels (12 500 copies per mL).
In the trial, she experienced 12 infections through year 5 (<2 per year discrete episodes) and efficiently cleared them. As a schoolteacher, she has chronic exposure to antigens. P3 contracted SARS-CoV-2 in year 5 but had mild symptoms (cough and rhinorrhea) that resolved. Her highest EBV levels during the extension (∼2000 copies per mL) were clinically significant but no infection was observed.35,36 Lymphopenia resolved by completion of the DFT. Her anxiety is ongoing, but she discontinued her medication and regularly visits her therapist. Over the trials, she received 17 prescription medications; at year 6, she received 7, with pulmonary medications prescribed as needed. P3 visits her immunologist and pulmonologist with decreased frequency.
Besides infections, P3’s AEs included sunburn, rash, hair loss, seborrheic dermatitis, oral ulcer, myalgia, weight gain, and temporomandibular joint pain. All were grade 1/2 and resolved, except for weight gain and myalgia.
During the study, P3 worked a part-time job, graduated from college, and started working full-time as a schoolteacher. She became more assertive, remained active, and expanded her circle of friends.
P4
P4 is 27-year-old male. He experienced recurrent infections that occasionally required hospitalization, failure to thrive, low body mass index, anemia (111 g/L; normal range, 130 to 175 g/L), and lymphopenia (0.5 × 109/L; normal range, 0.8 × 109/L to 3.3 × 109/L). He was unable to walk up a flight of stairs or engage in long conversations without dyspnea. P4 contracted infectious pneumonia and developed bronchiectasis (supplemental Figure 5). Before receiving leniolisib, he discontinued IRT and has not restarted.
After trial entry, P4 experienced only 1 AE: Lyme disease (grade 1) in year 1, which resolved. His anemia resolved within 6 weeks of the extension, and lymphopenia improved through year 6 (0.85 × 109/L; normal range, 1.32 × 109/L to 3.57 × 109/L). He experienced neutropenia shortly after the OLE started (1.26 × 109/L; lower limit of normal, 1.5 × 109/L) that resolved around year 2. His infectious pneumonia improved within a year, but infiltrates were present in his left lower lobe. His bronchiectasis did not progress and was stable at year 6. Although 5 medications were prescribed over the course of the study, P4 regularly took 3, which decreased to 2 by year 6. P4 gained weight and has had no hospitalizations.
P4 completed school before the trials and decided not to pursue higher education or work. Since receiving leniolisib, breathing improved and he can walk up to 15 miles without difficulty. P4’s overall demeanor and outlook on life noticeably improved. Family members noted differences in his physical capacities and increased socialization after 6 months of treatment.
P5
P5 is a 32-year-old male. P5 experienced arthralgia with edema of the joints, repeated airway infections, shortness of breath, and dermatitis. He had lymphopenia (0.6 × 109/L; normal range, 0.8 × 109/L to 3.3 × 109/L) that resolved by year 2. He was diagnosed with Hodgkin lymphoma at age 11 years and achieved full remission before receiving leniolisib.
While on treatment, P5 had 8 relapses of respiratory infections. His IRT dose reduced from 15 to 10 g/mo in year 6. P5 clears acute infections quicker, from 14 days to ∼3. During year 5, he contracted SARS-CoV-2 with a 1-day fever; symptoms resolved within the week. His dermatitis and joint pain resolved. Respiratory and gastrointestinal conditions improved. Over the trials, he received 22 prescription medications; at year 6, he received 7, of which the investigator deemed 3 crucial. Bronchiectasis that developed before the trials remained stable out to year 6 (supplemental Figure 6). He is only monitored by his pneumologist, and visits have decreased. Frequency and severity of infections reduced; prophylactic antibiotics ceased.
Besides infections, P5’s AEs included pruritis, sore throat, seborrheic dermatitis, diarrhea, productive cough, anxiety, noncardiac chest pain, petechiae, bilateral paresthesia of the fingers, hypercholesterolemia, xerostomia, stomach ache, and hepatopathy. All were grade 1 and resolved, except for COVID-19–associated mild dyspnea, hypercholesterolemia, and hepatopathy, with the latter 2 improving.
Positive changes in P5’s mood were noted as symptoms improved. P5 did not report any changes in shortness of breath; however, he has bronchial stenosis from lymphoma chemotherapy in 2011. He works a full-time labor-intensive job.
P6
P6 is a 39-year-old male. P6 experienced recurrent respiratory tract infections and was on a constant regimen of antibiotics. Fatigue negatively affected his exercise tolerance: he was unable to work or perform daily activities because of loss of energy. P6 had thrombocytopenia (123 × 109/L; normal range, 140 × 109/L to 370 × 109/L). He was diagnosed with mucosa-associated lymphoid tissue lymphoma at age 20 and achieved full remission before receiving leniolisib.
Throughout the trial, P6 experienced periodic infections through year 4. He had 2 confirmed cases of SARS-CoV-2, 1 in year 5 with mild rhinitis, and another in year 6. After 2 years of titration, P6 discontinued IRT at year 4; total IgG levels remained WNLs through year 6. After IRT termination, P6 experienced 4 mild infections through year 6. The number and severity of infections and need for prophylactic antibiotics declined. Thrombocytopenia resolved around year 1 (173 × 109/L; normal range, 150 × 109/L to 370 × 109/L). Over the trials he received 14 prescription medications; by year 6, he received 2 and no longer required recurrent antibiotics. P6 visits an immunology-pulmonology clinic yearly and remains under the care of his otolaryngologist, for which visits declined.
Besides infections, P6’s AEs included hearing loss, epistaxis, diarrhea, headache, inflammation at bone-anchored hearing aid, jaw pain, sore throat, tickling cough, painful wrist, reflux esophagitis, and Fuchs heterochromic iridocyclitis. All were grade 1/2 and resolved.
Since receiving leniolisib, P6 displays a more relaxed demeanor. His fatigue resolved, allowing him to partake in activities with his children. Within 2 months of treatment, he returned to work and socialized after work hours.
Discussion
Patients with an IEI such as APDS report a lower perception of general health and overall well-being compared to healthy individuals.19,41-43 We report improvements in several aspects of HRQoL assessed by standardized instruments. Our clinician-reported questionnaire bolsters HRQoL improvements by addressing aspects not assessed in standardized tools, such as decreased treatment burden and doctor’s office visits.
Many of the positive changes reported in HRQoL can be supported by clinical improvements, such as an increased ability to fight infections and decreased need for antibiotics. Sustained changes in lymphocyte subsets, including decreased transitional B cells and increased CD4:CD8 T-cell ratio, suggest an improvement in immune deficiency and dysregulation. Most patients had stable immunoglobulin levels through year 6, including those who decreased dose or discontinued IRT. Changes in immune subsets may explain the reduction in both number and severity of infections reported in most patients receiving leniolisib, and patients who contracted SARS-CoV-2 experienced mild symptoms.
Regarding lung disease, bronchiectasis in all 3 affected patients showed no progression. More than half of the cytopenias that were present during treatment across the trials resolved by year 6. Patients whose cytopenias did not fall WNLs improved; notably P2 had a marked increase of platelets from 53 × 109/L to 148 × 109/L by year 6.
This study has several limitations. Initially, patients visited trial investigators every 2 to 3 months; time between visits increased to every 6 to 7 months after year 2, during which time patients may have experienced symptoms that were not reported at their next visit. Additionally, there was a treatment gap between the dose-finding and extension trials, with 3 patients not receiving leniolisib for 25 to 50 days, and 2 patients not receiving leniolisib for ∼1 year, during which time manifestations may have gone unreported. Small sample size and lack of a placebo-controlled arm also limit generalizability. Lastly, the subjective portion of the clinician-reported questionnaire is subject to recall and investigator bias. Furthermore, we acknowledge that data captured in our clinician-reported questionnaire are subjective by nature and limits the general applicability of data that were generated from it. For instance, infection susceptibility data had no standardized protocol for collection because it was not a primary outcome measure; it was collected per site and evaluated at investigator discretion. Moreover, several years of data collection occurred during the COVID-19 pandemic, during which most evaluations were completed remotely.
In addition to these limitations, gaps in knowledge remain. The rate of malignancy in patients treated with leniolisib compared with those not treated warrants further investigation. Early onset of APDS can be a risk factor for developing severe disease, and accordingly, the safety and efficacy of leniolisib is being examined in 2 pediatric trials (ClinicalTrials.gov identifiers: NCT05693129 and NCT05438407). Questions remain around the use of leniolisib as a bridge to hematopoietic stem cell transplant. Risks such as graft failure should be considered during the decision-making process.44,45
Remarkably, within 10 years of the discovery of APDS, leniolisib was developed as a novel and selective treatment option.1,46 The mechanism of action of leniolisib precisely matches the molecular underpinnings that cause manifestations of APDS.1,6 Our previous work has shown long-lasting improvements in immune subsets and lymphadenopathy in patients with APDS who received leniolisib.20-22
Here, we had the unprecedented opportunity to examine HRQoL and clinical outcomes in adult patients with APDS exposed to leniolisib for 6 years. Overall, we observed positive and enduring changes in both, with minimal toxicity, supporting the use of leniolisib as a long-term precision therapy for the treatment of APDS in appropriate patients.
Acknowledgments
The authors thank the patients who took part in the study and their families. Alexander Ling (Radiology and Imaging Sciences, National Institute of Health and Clinical Center, Bethesda, MD) provided computed tomography scans for the patients of the National Institutes of Health. The authors thank Carolyn Keating, Jaclyn DeFinis, and the staff at PRECISIONscientia for data collation and manuscript preparation.
Novartis Pharmaceuticals and Pharming Group, NV provided funding for the clinical trials, and Pharming Healthcare, Inc, provided funding for the manuscript preparation by PRECISIONscientia.
The opinions expressed in this article are the authors’ own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the US government.
Authorship
Contribution: V.K.R., E.K., S.W., A.Š., V.A.D., and G.U. performed research and collected data; J.G., D.B., and H.C. are the local recruiters of patients involved in the study, and provided feedback during manuscript development; and J.B. was involved in oversight of the data reports.
Conflict-of-interest disclosure: E.K. is an employee of Leidos Biomedical Research, Inc. S.W. is a consultant for Pharming Group, NV. A.Š. is a consultant for Octapharma, Takeda, and Pharming Group, NV. V.A.D. is a consultant for, and/or receives honoraria from, AstraZeneca, Kedrion, Takeda, CSL Behring, Pfizer, and Pharming Group, NV, and receives research funding from Takeda. J.B. is a current employee and stock option holder of Pharming Group, NV and holds individual stock in Neoclone. The remaining authors declare no competing financial interests.
Correspondence: V. Koneti Rao, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Room 10/12C106, 10 Center Dr, Bethesda, MD 20892; email: koneti@nih.gov.
References
Author notes
Pharming is committed to responsibly share data generated by interventional clinical trials with researchers; individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendices), may be shared; data will be made available beginning 12 months and ending 36 months after publication of this article; researchers who have a methodologically sound research proposal should send inquiries or requests to info@pharming.com; data requestors may be required to sign a data access agreement.
The full-text version of this article contains a data supplement.