Key Points
The benefits and risks of gene therapy are currently unclear, so it is key to understand their potential value from the patients’ perspective.
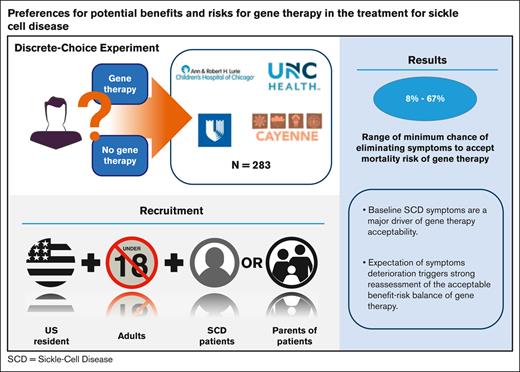
The appeal of gene therapy is evident with most respondents, although those with milder symptoms see other treatment options as viable.
Abstract
Objective of this study is to quantify benefit-risk tradeoffs pertaining to potential gene therapies among adults and parents/caregivers of children with sickle cell disease (SCD). A discrete-choice experiment survey was developed in which respondents selected their preferred treatment alternatives in a series of experimentally controlled pairs of hypothetical gene therapies and a “no gene therapy” option. Gene therapy alternatives were defined based on the chance of eliminating SCD symptoms, expected increases in life expectancy they could offer, treatment-related risk of death, and potential increases in lifetime cancer risk. Respondents made selections based on their current disease severity and in the context of expectations of worsened disease. Three clinical sites and 1 patient organization recruited 174 adult patients and 109 parents of children with SCD to complete the survey. Adult and parent respondents were generally willing to choose gene therapies, but the adults required higher expected levels of efficacy (ie, higher chance of eliminating symptoms) than parents to choose gene therapies that conferred mortality risks of ≥10%. When adults and parents of children with less severe symptoms were asked to consider scenarios of higher levels of disease severity, the increased risk tolerance, and the lowest acceptable level of efficacy for gene therapies with mortality risks dropped by >50%. Baseline SCD symptoms are a major driver of gene therapy acceptability. Adults and parents of patients with milder symptoms may prefer other treatment options; however, an expectation of symptoms deterioration triggers strong reassessment of the acceptable benefit-risk balance of this novel technology.
Introduction
Sickle cell disease (SCD), a rare blood disorder caused by a mutation in the β-globin gene, affects ∼100 000 people in the United States, and is found predominantly in Black or African American communities.1,2 Although survival rates are improving, >90% of children with SCD in countries with poor resources do not live past 18 years of age, and the burden of SCD continues to grow globally.3,4 People with SCD often experience vaso-occlusive crises (VOCs), chronic anemia, acute chest syndrome (ACS), stroke, and organ damage. These outcomes affect quality of life negatively and lead to hospitalizations and increase mortality.5 Currently approved pharmacological treatments for SCD include hydroxyurea, voxelotor, L-glutamine, and crizanlizumab.6 These pharmacological treatments have different mechanisms of action and may alleviate symptoms, but are not curative in nature.6 Allogeneic hematopoietic stem cell transplantation (HSCT), a potentially curative option, is dependent on patients having a matched donor and can be associated with serious immune-related complications such as graft-versus-host disease, which may be life-threatening.7 Another option, haploidentical HSCT, has become increasingly available and could help avoid the need for a matched donor. However, it carries a higher risk of rejection.8,9 Gene therapy approaches, currently under clinical investigation, are emerging as a promising potential disease-modifying treatment for SCD, because they do not require finding a matched donor and avoid immune-related complications associated with allogeneic transplants.10-13 There are several ongoing clinical trials investigating the use of gene therapies in SCD.14
Incorporating the patient experience into health care decision-making is increasingly being valued. Recent guidance documents on benefit-risk assessment issued by the US Food and Drug Administration describe how patient experience or patient input data can support benefit-risk assessments.15,16 In particular, patient preference information can provide insights into how patients value benefits in comparison to risks, and can be especially useful when patients’ views about the most important benefits and risks are expected to vary considerably.15-17 Given that gene therapy in SCD is novel and the benefit-risk profile of the treatment is currently unclear, it is important to understand SCD patients’ acceptance of gene therapy and the relative value that they place on the potential benefits and risks associated with this technology. Furthermore, it is important to consider the possibility that patients’ views and risk tolerance regarding gene therapy may differ according to their disease severity. For instance, patients whose disease severity is on the higher end of the spectrum may be more accepting of the risks of a novel gene therapy than patients whose disease is well-controlled on currently available therapies.
Although there are studies describing SCD patients’ knowledge and beliefs about gene therapy,18,19 literature on patients’ evaluation of the benefit-risk profiles of gene therapies remains limited. This study aimed to elicit the benefit-risk preferences that adult patients and caregivers of pediatric patients have for gene therapy in SCD and to quantify their willingness to accept gene therapy under various scenarios. The study was also designed to evaluate the impact of SCD severity on the acceptability of outcomes associated with gene therapy. Such information not only would offer preliminary insights into tradeoffs that patients could consider acceptable when gene therapy becomes more widely available, but also could serve as a way to contextualize trial results in this disease area from the patient and caregiver perspectives.
Methods
A discrete-choice experiment (DCE) survey instrument was developed, tested, and fielded to quantify the perspective of US adult patients with SCD and parents of pediatric patients with SCD on potential outcomes of gene therapy for SCD. The study, including the survey instrument, was designed following best practices outlined by stated-preference experts and recommendations in the US Food and Drug Administration guidance on patient preference information.20-22
In the DCE, respondents were asked to select their preferred treatment alternative between experimentally controlled pairs of hypothetical gene therapies along with a “No Gene Therapy” option. The “No Gene Therapy” profile indicated the baseline outcomes for patients receiving standard of care without gene therapy. Without gene therapy, patients were told that they should expect that the symptoms that they had in the 12 months before the survey would continue for the purpose of the decision and that they would not experience any increase in life expectancy or any additional risk associated with gene therapy.
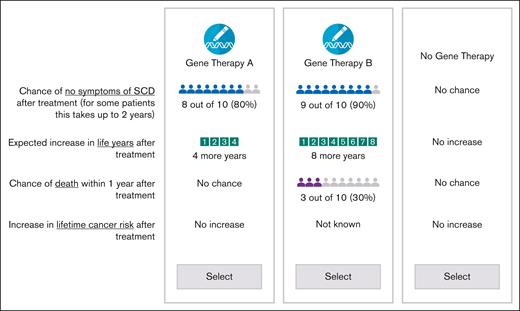
The gene therapies were defined by characteristics (or attributes) related to the efficacy (chance of no symptoms of SCD after treatment and expected increase in life expectancy after treatment) and risks of adverse events from treatment, which included a risk of death and a potential increase in lifetime risk of cancer (Table 1). These attributes and levels were selected based on results from an in-person focus group with 4 parents of pediatric patients and 4 adult patients and were presented to facilitate assessing treatment preferences across respondents in a standardized manner. Additional details on the selection of the study attributes and attribute levels can be found in the section describing the development of study attributes in supplemental Material Appendix A.
To prepare respondents for the choice questions, the survey instrument included descriptions of all attributes, comprehension questions, a tutorial for probabilistic attributes, and simplified practice choice questions. Before fielding the final survey, one-to-one pretest interviews with 9 adult patients and 7 parents of pediatric patients were conducted to obtain feedback on the survey instrument. The team tested the plausibility of various gene therapy scenarios and made revisions to the survey content based on the feedback received. The levels of the attributes were selected and revised such that there were sufficient ranges of compensatory benefits and risks to encourage most participants to accept tradeoffs across choice questions. Upon completion of the interviews, the team finalized the survey instrument (survey section in supplemental Material Appendix B) and the layout of the choice questions (Figure 1).
Gene therapies were populated using an experimental design. “No Gene Therapy” option was fixed across choice questions.
Gene therapies were populated using an experimental design. “No Gene Therapy” option was fixed across choice questions.
Respondents first answered either 4- or 6-choice questions based on the current self-reported symptom severity and summarized according to a symptom-severity scale developed by Shah et al,23 including the number of pain crises that required treatment at a hospital or clinic, chronic pain lasting at least 6 months and presence of a stroke or ACS in the 12 months before the survey.
Patients were considered to have mild SCD symptoms (group A) if they reported ≤1 pain crises in the past 12 months and no chronic pain, and no stroke or ACS. Patients and parents of children with mild SCD symptoms were expected to answer the first 4-choice questions in regard to their baseline condition. The rest of the respondents (group B) answered the first 6-choice questions considering their baseline condition. Patients in group B were those who reported any of the following 3 symptoms: (1) ≥2 pain crises in the last 12 months, (2) chronic pain for at least 6 months, and (3) stroke and/or ACS. Group A respondents (ie, milder baseline symptoms) were asked fewer choice questions (ie, 4 instead of 6) initially because in the pretest interviews these patients were more likely to opt out of gene therapy. Thus, little or no information was expected to be available on attribute preferences from these respondents reporting milder symptoms, regardless of the number of questions asked.
All respondents answered the remaining choice questions (group A: 9; group B: 7) by supposing their condition worsened in particular ways (“worsened” state). Therefore, each respondent answered 13 questions in total. Respondents who reported having milder symptoms (group A) were asked to suppose that they had experienced chronic pain for 6 months, had ≥5 hospital visits to handle pain crises within a 12-month period, and experienced a stroke without long-term sequelae (ie, transient ischemic attack) (Table 2). Respondents in group B were told to suppose they experienced ≥5 pain crises in a 12-month period and 6 months of chronic pain. In addition, if respondents from group B did not report experiencing any stroke or ACS at baseline, they were told to suppose that they experienced a stroke without long-term sequelae. If they reported a stroke at baseline, they were told to suppose that they experienced a debilitating stroke with long-term sequelae.
In general, the experimental design for a DCE determines the combinations of attribute levels that define each hypothetical treatment profile in the choice questions. Following good research practices, our DCE design was generated using design macros developed for SAS 9.4 (Cary, NC) based on D-efficiency.21,24 Details on the design are presented in supplemental Figure C1 (Appendix C). Additional comprehension questions and internal-consistency checks were also included to evaluate the quality of the data included, choice patterning, attribute dominance, across-set monotonicity, and a scope test (experimental design section in supplemental Material Appendix C).
The web-based surveys for the adult and parent respondents were programmed and administered using Lighthouse Studio (Sawtooth Software, Inc, Provo, UT). The study protocol was reviewed and approved by the Institutional Review Board of all participating academic institutions.
Sampling framework
Adults and parents of children with SCD were recruited through the Duke University Health System, the University of North Carolina at Chapel Hill, Lurie Children’s Hospital of Chicago, and Cayenne Wellness, a patient advocacy group based in California. Respondents had to be able to read English and willing to provide consent to the research. Participants recruited through Cayenne Wellness were required to self-report a physician diagnosis of SCD for themselves and their children. Adult patients who also had children with SCD were randomized to either the patient (ie, answer the survey assuming they could get gene therapy for themselves) or parent version (ie, answer the survey assuming they could get gene therapy for their child and given their child’s condition). Parents with multiple children with SCD were asked to consider the child with the worst current symptoms as they completed the survey. Participants were compensated $15 for taking part in the survey.
Analysis
DCEs generate data that relate respondents’ choices to the differences in the attribute levels across alternatives. The statistical analysis of choices provides a measure of the impact of changes in the attribute levels on the likelihood that treatments are selected. Results represent log-odds preference weights and indicate the relative preference for treatments with specific attribute levels, all else equal.22 Higher preference weights indicate greater chance of choosing a treatment with such characteristics.
Heteroskedastic logit models were used to evaluate scale (ie, variance) differences across recruitment sources and to determine whether choice data could be pooled.25 Population-level preferences were estimated separately for adult patients and parents of patients using random-parameters logit (RPL) regression models. Preferences for all attributes were allowed to vary across respondents using normally distributed random parameters. Correlation effects across attributes were included to account for scale effects unrelated to data sources.
Based on model fit, linear specifications were selected for all numerical attributes except the chance of death in the adult-patient sample. Expected lifetime risk of cancer was modeled using dummy-coding, in which the omitted level represented no increased risk of cancer. Finally, we tested for possible interaction effects between the chance of no symptoms of SCD and increases in life expectancy.
Preference weights from the fully correlated RPL models were normalized to facilitate comparisons between the adult-patient and parent samples.26 Treatment efficacy was rescaled between 0 and 10, where 0 corresponded to no gene therapy and no efficacy. All the other attributes and levels were rescaled accordingly to preserve the relative effects across attributes.
Preference-weight estimates from each RPL model were used to compute minimum-acceptable benefits (MABs). MABs represent the minimum chance of eliminating SCD symptoms with gene therapy that would leave the patient at least as well off as they would be without gene therapy given a specific treatment-related increase in the risk of death. To compute the MABs, we calculated the disutility associated with a gene therapy that implied a specific increase in the risk of death. We contrasted the value of that gene therapy against the option of not getting gene therapy and accepting a continuation of the SCD symptoms. Thus, the perceived acceptability of the mortality risk with gene therapy was affected by the severity of the baseline symptoms. We then determined the increase in the chance of eliminating SCD symptoms that would offset the potential harm of the gene therapy. Confidence intervals (CIs) were calculated using Krinsky and Robb parametric bootstrap simulations27 based on the model variance-covariance matrix for mean estimates.
Results
Of the 266 and 114 adult patients and parents of pediatric patients screened, 180 (68%) and 114 (100%) met the study inclusion or exclusion criteria, respectively. The DCE survey was completed by 178 (99%) adult patients and 111 (97%) parents. Four adult patients and 2 parents were regarded as inattentive to the content of the choice questions; that is, they always chose “Gene Therapy A” or “Gene Therapy B” across all 13 questions and were excluded from data analysis. Table 3 reports characteristics of the study samples representing 174 adult patients and 109 parents. The average age of adult patients was 34 years. Approximately 73% were female, 97% were Black or African American, and 40% held at least a bachelor’s degree. The average age of parents was 40 years. Approximately 79% were female, 94% were Black or African American, and 31% held at least a bachelor’s degree.
Adult patients reported an average of 3 pain crises that required medical attention in the 12 months before the survey. Fifty-eight percent patients experienced some level of continued pain for at least 6 months. Approximately 20% of the patients had an ACS episode at some point in the past. Seven adults (4%) reported having had a stroke in the 12 months before taking the survey, of which 2 resulted in permanent mental disability and/or physical disability.
The average age of the pediatric patients was ∼9 years, and 47% were female. Parents of these patients reported that their children had experienced an average of 1 pain crisis that required attention at a hospital or a clinic in the 12 months before the survey. Nineteen percent of the children had suffered some level of continued pain for at least 6 months or an ACS episode. Less than 1% of the children had had a stroke, none of whom experienced permanent mental or physical disability. However, when asked to recall the child’s worst year with SCD symptoms, caregivers reported their child experienced an average of 3.8 VOCs that required hospitalization. Approximately 18% (18.3%) of parents also reported having SCD. Finally, ∼88% (87.7%) of parents stated that they expected their child with SCD would live at least as long as the average life expectancy provided for this population, and ∼70% (69.8%) stated they thought their child would live longer.
Of the 4 comprehension questions in the survey instrument, 85% of adult patients and ∼82% of parents provided ≥3 correct answers. Less than 2% of respondents made their choices based solely on 1 attribute (ie, attribute dominance). Approximately 2.8% of adult patients and 1.8% of parents always chose a gene therapy. Meanwhile, ∼6.9% of adult patients and 4.6% of parents always chose to opt out. Approximately 10% of respondents failed across-set monotonicity checks (10.7% and 10.8% for adult patients and parents, respectively). With regard to the scope test included in the DCE, we found that it is possible to reject a scope-test failure among adult patients (P = .05), but the same was not true of parents (P = .32). Although these evaluations help ascertain the consistency of responses in the data, there may be valid reasons for patterns of choices that do not meet a priori expectations.28
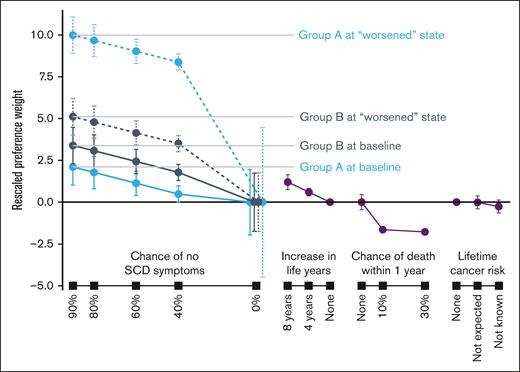
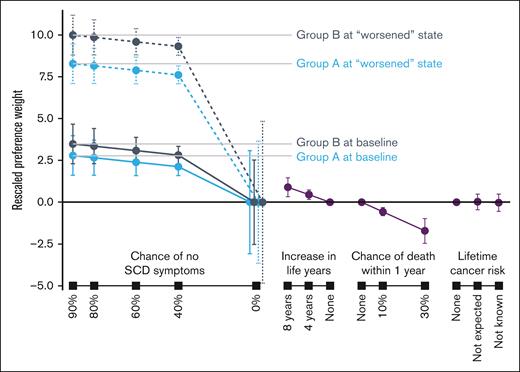
Preference weights from the RPL models are reported in supplemental Tables D1 and D2 (Appendix D). Rescaled preference weights for the adult and parent samples are shown in Figures 2 and 3. In general, better clinical outcomes were preferred (ie, higher preference weights). Gene therapies with greater chances of no SCD symptoms or greater improvements in life years were preferred by both adult patients and parents. Both adult patients and parents preferred lower chances of death within 1 year after treatment. When considering gene therapies that resulted in an increase in lifetime cancer risk, neither adult patients nor parents discriminated among the attribute levels presented.
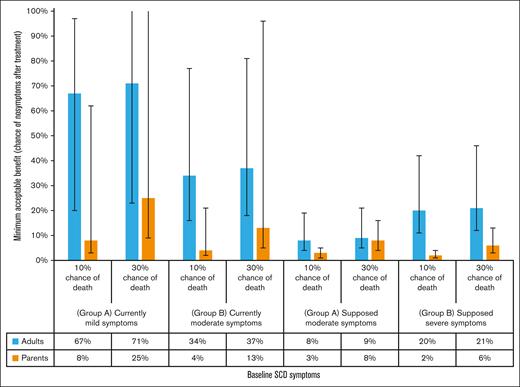
Group A at baseline represented respondents reporting symptoms and disease history consistent with mild SCD severity. Group A at “worsened” state was asked to assume having chronic pain for 6 months, ≥5 hospital visits to handle pain crises, and a transient ischemic attack (mini stroke). Group B at baseline represented respondents reporting symptoms and disease history consistent with moderate or severe SCD severity. Group B at “worsened” state was asked to assume having (1) chronic pain for 6 months, ≥5 hospital visits to handle pain crises, and a transient ischemic attack (stroke) if they never had a stroke or ACS; or (2) chronic pain for 6 months, ≥5 hospital visits to handle pain crises, and a debilitating stroke with long-term sequelae if they had a stroke or ACS in the past. Of the 174 respondents, 44 and 130 respondents were in group A and group B, respectively. Error bars indicate 95% CIs.
Group A at baseline represented respondents reporting symptoms and disease history consistent with mild SCD severity. Group A at “worsened” state was asked to assume having chronic pain for 6 months, ≥5 hospital visits to handle pain crises, and a transient ischemic attack (mini stroke). Group B at baseline represented respondents reporting symptoms and disease history consistent with moderate or severe SCD severity. Group B at “worsened” state was asked to assume having (1) chronic pain for 6 months, ≥5 hospital visits to handle pain crises, and a transient ischemic attack (stroke) if they never had a stroke or ACS; or (2) chronic pain for 6 months, ≥5 hospital visits to handle pain crises, and a debilitating stroke with long-term sequelae if they had a stroke or ACS in the past. Of the 174 respondents, 44 and 130 respondents were in group A and group B, respectively. Error bars indicate 95% CIs.
Group A at baseline represented respondents reporting symptoms and disease history consistent with mild SCD severity. Group A at “worsened” state was asked to assume having chronic pain for 6 months, ≥5 hospital visits to handle pain crises, and a transient ischemic attack (mini stroke). Group B at baseline represented respondents reporting symptoms and disease history consistent with moderate or severe SCD severity. Group B at “worsened” state was asked to assume having: (1) chronic pain for 6 months, ≥5 hospital visits to handle pain crises, and a transient ischemic attack (stroke) if they never had a stroke or ACS; or (2) chronic pain for 6 months, ≥5 hospital visits to handle pain crises, and a debilitating stroke with long-term sequelae if they had a stroke or ACS in the past. Of the 109 respondents, 56 and 53 respondents were in group A and group B, respectively. Error bars indicate 95% CIs.
Group A at baseline represented respondents reporting symptoms and disease history consistent with mild SCD severity. Group A at “worsened” state was asked to assume having chronic pain for 6 months, ≥5 hospital visits to handle pain crises, and a transient ischemic attack (mini stroke). Group B at baseline represented respondents reporting symptoms and disease history consistent with moderate or severe SCD severity. Group B at “worsened” state was asked to assume having: (1) chronic pain for 6 months, ≥5 hospital visits to handle pain crises, and a transient ischemic attack (stroke) if they never had a stroke or ACS; or (2) chronic pain for 6 months, ≥5 hospital visits to handle pain crises, and a debilitating stroke with long-term sequelae if they had a stroke or ACS in the past. Of the 109 respondents, 56 and 53 respondents were in group A and group B, respectively. Error bars indicate 95% CIs.
Proclivity for gene therapy can be evaluated through the relative preference weights for positive chances of eliminating SCD symptoms. The distance between these weights and the weight for 0% chance of eliminating symptoms conveys the intensity of preference for treatment. Adult patients in group A (ie, mild symptom severity) were less likely to accept gene therapy at current baseline compared to group B adults. Interest in gene therapy increased substantially as adult respondents in group A were asked to suppose they experienced worse symptoms (ie, had chronic pain, ≥5 pain crises and a stroke without long-term sequelae). Adult patients in group B were more likely than those in group A to accept gene therapy with any of the efficacy levels presented in the choice questions (Table 1). Among parents of patients, we found no difference in their proclivity for gene therapy across current baseline levels (ie, group A at baseline vs group B at baseline). The likelihood of choosing gene therapy with the supposed worsened state increased more for group B than group A among parents, and more for group A than group B among adult patients.
Figure 4 reports the MABs required of gene therapies with 10% and 30% chances of death within 1 year after treatment by adult patients and parents. The MABs corresponded to respondents’ choices assuming their current symptoms and with “worsened” symptoms. To accept a 10% chance of death, adult patients with their current symptoms in group A required a MAB of 67% (95% CI, 20-97) whereas those in group B only required 34% (95% CI, 16-77). At the “worsened” state, the MAB required by group A decreased to 8% (95% CI, 4-9) whereas group B required a 20% MAB (95% CI, 11-42).
Parents required lower MABs compared with adult patients. For group A, the MABs for a treatment with a 10% chance of death were 8% (95% CI, 3-62) given the patients’ current symptoms and 3% (95% CI, 1-5) for the “worsened” state. For group B, the MABs were 4% (95% CI, 2-21) given the patients’ current symptoms and 2% (95% CI, 1-4) for the “worsened” state. Similar patterns in MABs and greater MABs were expected for accepting a 30% chance of death.
Discussion
This is the first study investigating adult patient and parent preferences for outcomes associated with gene therapy for SCD. Our study is also unique with its within-respondent evaluation of choices in the context of current and worsened disease-related severity. We found that adult patients and parents were generally willing to opt for gene therapies, but they have different perspectives on the acceptability of their benefit-risk tradeoffs. Preferences were logically ordered for both adult patients and parents. Both adult patients and parents more often opted for gene therapies offering higher chances of eliminating SCD symptoms and longer life expectancy, and less often opted for gene therapies with higher chances of death, controlling for other factors.
Adults experiencing mild SCD symptoms required gene therapies to offer at least a 67% chance of eliminating SCD symptoms, on average, to accept a 1-year mortality risk of 10% associated with gene therapy. In contrast, adults experiencing moderate symptoms required much lower levels of benefit. For example, to accept gene therapies posing 10% or 30% mortality risks, adult patients required 34% and 37% chance of no SCD symptoms, respectively.
Although a 30% mortality risk was far beyond the known risks for gene therapy at the time of this survey, the inclusion of such a high level of risk allowed us to conclude that gene therapies offer benefits that are potentially much more valuable than the negative impacts they entail. Respondents’ willingness to consider treatments with such a high level of mortality risk is consistent with feedback provided by caregivers and patients during the interviews to test the survey instrument. Thus, although the inclusion of a 30% mortality risk in the DCE is not meant to imply that such a risk level is acceptable in the context of clinical decision making, it is a reflection of the great importance of potentially curative therapies.
Disease severity was observed to have a large influence on benefit-risk preferences for gene therapy. When adult patients who were experiencing mild symptoms considered worsened SCD severity, they required less benefits (ie, ∼8% chance of eliminating SCD symptoms, given a 10% chance of dying from the treatment). Adults who reported experiencing moderate symptoms required ∼20% chance of eliminating SCD symptoms for gene therapies with a 10% mortality risk when asked to consider having worsened symptoms.
Similar patterns regarding efficacy requirements were observed when parents were asked to consider more severe symptoms than their child’s current symptoms. However, overall MAB estimates given gene-therapy risks were much lower among parents compared with adults regardless of baseline symptoms. These high levels of risk tolerance are consistent with previous documented results for parents of patients when evaluating acceptability of hematopoietic stem cell transplant transplant in SCD.29 In 1 study, ∼40% of parents who were interviewed said that they would be willing to accept at least a 15% chance of death from the procedure, and 12% stated that they would be willing to accept >50% chance of treatment-related mortality.30 Nonetheless, more studies are needed to confirm these levels of risk tolerance and to gain a broader understanding of their reasoning.
In general, greater acceptance of gene therapies was observed when respondents were asked to consider a higher level of disease severity, suggesting higher risk tolerance with more advanced disease states. The relatively lower levels of acceptance among those who actually experienced more severe symptoms suggests adaptation as they gain experience with new or more severe symptoms. This observation is common in other chronic diseases where patients report good quality of life despite a lower rating of health states by observers.31 We also note that the CIs around the estimates for no gene therapy were much wider in the questions where respondents had to imagine worsened symptoms. Taken together, caution may be warranted when asking patients about their treatment preferences for a health state that they have not yet experienced. One exception to this pattern was seen among parents of patients evaluating worsened baselines. It is possible that parents of young patients discounted the difference in the severity of strokes based on their expectation that stroke screening and preventative measures would likely prevent serious sequelae.
Several factors may be contributing to the high-risk tolerances observed in our study. First, it is possible that respondents considered the level of uncertainty around efficacy measures. In the survey, we presented scenarios of efficacy and risks out of 10 patients treated with gene therapy. Respondents could have reasonably surmised that the level of efficacy could vary dramatically as the number of patients treated increased beyond 10. This is potentially a real issue given the currently limited evidence collected through trials for gene therapy. Second, respondents in group B who reported that the patient experienced a stroke at baseline were asked to suppose the patient experienced another stroke with sequelae. Previous work suggests such an event is very impactful, and potentially more so than death.32 Third, media attention to successful gene-therapy interventions around the time of data collection could have triggered some level of hope for better outcomes among respondents. Finally, caregivers could have been reacting to the issues experienced when their child’s SCD symptoms were most severe. Although short-term survival among children with SCD is not as big of a concern, the stress and pain of VOCs could have led caregivers to focus on avoiding serious quality-of-life issues through gene therapy.
Although the risk of death from gene therapy was considered important by respondents, the potential increase in the lifetime risk of cancer was not perceived as important relative to the other study attributes. Changes between the levels for this attribute may be too small to be statistically significant given the study sample size (<180). The qualitative description of the attribute might have played a role as there is some evidence that ambiguity in the definition of adverse effects can reduce their importance.33 Furthermore, the prognosis of cancers can be variable depending on the type of cancer and this also may have contributed to the ambiguity of the definition. Furthermore, we informed respondents in the survey that the lifetime probability of cancer without gene therapy is about 1 in 3, which is consistent with the lowest estimated probability for adults in the United States.34
Limitations include the fact that choices made based on hypothetical scenarios do not carry the same consequences as real-world choices. Furthermore, part of the study required respondents to imagine that they experienced a worsened health state, which may be difficult for the respondents to do. Although we tried to provide specific descriptions of the symptoms that are experienced with the worsened health state in the survey, there may still be variation among how respondents interpreted or imagined a worsened health state. Although this approach has its limitations and compounds the hypothetical nature of the decisions elicited in the survey, it provided a way to evaluate the impact of worsening baseline conditions. In that sense, our study provides insights into decision making among individuals with conditions that can be expected to worsen in the future. Our results suggest there is a heightened need for attention to patient education and consent as patients’ conditions worsen.
Another limitation is that some respondents had to self-report the severity of the symptoms and we cannot ascertain with certainty if what they reported was reflective of their actual clinical severity. Nonetheless, the questions used in the survey to classify baseline severity were reviewed by clinical experts and further tested with patients during in-person interviews before fielding the survey. Furthermore, we used a convenience sample for this study and the sample may not be completely generalizable to the US SCD population. And yet, our study sample generally reflects greater prevalence of chronic pain and disability among adults compared to children with SCD. Although pediatric patients can have serious and life-threatening complications, their incidence has decreased compared to historical levels owing to effective screening and prevention measures.35 Thus, our study would provide preliminary insights of risk tolerance toward gene therapy, especially among caregivers of pediatric patients who are less likely to manifest the chronic complications of SCD associated with end organ damage and have lower health care use.
Finally, our survey included information about gene therapy based on knowledge available at the time of the study. Understanding of gene therapy outcomes is continually evolving and our study findings may not capture the full spectrum of differences between gene therapies and other treatment options. For example, a description of the possibility of infertility as a result of myeloablative conditioning was included, but we did not alter the likelihood of future fertility problems across treatment options. With currently used bone marrow preparative regimens for curative treatments, risk of infertility is expected to be comparable between gene therapy and HSCT, with variables such as age, menarchical status, and specific drugs used affecting outcomes.36 Nevertheless, understanding of fertility issues associated with gene therapy could evolve over time. Furthermore, when we designed the study, haploidentical HSCT was not widely accepted as a potential substitute for gene therapy. Thus, the study did not include it as a potential treatment option for patients with SCD. Finally, we used a qualitative description of the risk of malignancy associated with gene therapies and limited the long-term risk of death from gene therapies to this particular outcome. Although these factors could affect how patients and caregivers evaluate the benefits and risks of gene therapy, they were based on the evidence available at the time of survey implementation. As clinical evidence evolves, future stated-preference studies should be conducted to better elucidate acceptable benefit-risk tradeoffs with gene therapy for SCD. Future research also could focus on understanding where similarities and differences may lie in the acceptability of gene therapies among providers and with regard to the parental role of caregivers.
The decision to receive gene therapy is invariably a complex one and includes evidence that is evolving quickly, even from the time this survey was developed and administered. Nonetheless, this study provides the first rigorous evaluation of the acceptable tradeoffs associated with gene therapy, forms a basis for evaluating gene therapies from the perspective of adult patients and caregivers of pediatric patients, and contributes to ongoing discussions about the acceptability of this novel technology.
Conclusions
The appeal of gene therapy is evident in our study results, with respondents accepting gene therapy in most scenarios presented, despite the risks associated with these novel therapies. Although symptom severity can be expected to vary significantly throughout patients’ lives, severity of recent symptoms within the last 12 months appears to be a major driver of the acceptability of the risks associated with gene therapy. Not surprisingly, those with milder symptoms may be less willing to accept the risks of gene therapy and see other treatment options as viable. However, an expectation of rapid deterioration of health through organ damage and vaso-occlusive events can trigger strong reassessment of the acceptable balance between benefits and risks of this novel technology. As better risk stratification for these patients is developed in the future, it would be key to convey this information clearly to patients to enable decision-making regarding gene therapies.
Acknowledgments
The authors acknowledge the support of Mark Walters (University of California San Francisco) and Alexis Thompson (Children’s Hospital of Philadelphia). This work was supported by a contract with the US Food and Drug Administration (HHSF223201810163C). The content is solely the responsibility of the authors and does not necessarily represent the official views of the US Food and Drug Administration.
Authorship
Contributions: J.M.G.S. conceptualized the study, performed research, analyzed data, interpreted results, and wrote the manuscript; J.C.Y. performed research, analyzed data, interpreted results, and wrote the manuscript; S.D.R. conceptualized the study, performed research, interpreted results, and wrote the manuscript; T.-H.L. conceptualized the study, performed research, interpreted results, and wrote the manuscript; X.N. performed research, interpreted results, and wrote the manuscript; S.S. conceptualized the study, performed research, and interpreted results; T.I. conceptualized the study and performed research; M.H. conceptualized the study and performed research; J.A.R. performed research, interpreted results, and wrote the manuscript; S.B. performed research, interpreted results, and wrote the manuscript; C.R. performed research, interpreted results, and wrote the manuscript; J.L. performed research, interpreted results, and wrote the manuscript; N.R.S. performed research, interpreted results, and wrote the manuscript; K.L. performed research and analyzed data; and M.J.T. conceptualized the study, performed research, interpreted results, and wrote the manuscript.
Conflict-of-interest disclosure: T.I. is an employee at and receives salary and stocks from Janssen Pharmaceutical Companies of Johnson & Johnson. M.H. is an employee of and receives salary from Pfizer. This study was initiated and implemented when T.I. and M.H. were employees and T.-H.L. was a fellow with the US Food and Drug Administration, Center for Biologics Evaluation and Research. T.I. is currently working at the Janssen Pharmaceuticals Companies of Johnson & Johnson, Raritan, N.J. J.A.R. reports research funding from Agios Pharmaceuticals, Novartis, Pfizer, bluebird bio, Dova Pharmaceuticals, and consulting or advisory board membership for Agios. N.R.S. reports consulting work for GBT, bluebird bio, Forma, Agios, and Emmaus; speaker engagements supported by GBT, Emmaus, and Alexion; and research funding from GBT. The remaining authors declare no competing financial interests.
The current affiliation for S.S. and X.N. is the US Food and Drug Administration.
Correspondence: Juan Marcos Gonzalez Sepulveda, Duke University School of Medicine, 200 Morris St, Durham, NC 27701; e-mail: jm.gonzalez@duke.edu.
References
Author notes
Data are available upon request from the corresponding author, Juan Marcos Gonzalez Sepulveda (jm.gonzalez@duke.edu).
The full-text version of this article contains a data supplement.