TO THE EDITOR:
The treatment landscape for chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) continues to evolve as new therapies are evaluated in clinical trials. With the availability of novel, targeted agents, questions remain regarding optimal treatment selection for patients with CLL/SLL in the real-world setting.1,2 Treatment guidelines for CLL/SLL have expanded in recent years with a greater understanding of the importance of prognostic biomarker testing to inform treatment selection.3-8 The 2018 International Workshop on Chronic Lymphocytic Leukemia (iwCLL) recommends interphase fluorescence in situ hybridization (FISH) testing to detect chromosomal abnormalities and sequencing for tumor protein p53 (TP53) mutations and for immunoglobulin heavy chain variable (IGHV) somatic hypermutation status,8 which are critical for risk stratification. Patients with high-risk genomic features are more likely to have poor outcomes when treated with chemoimmunotherapy (CIT) and should be considered for targeted therapies.8-10
Here, we present real-world prognostic testing rates, treatment selection, and treatment patterns over time in the fully enrolled population from the informCLL registry. Moreover, with the coronavirus disease 2019 (COVID-19) pandemic emerging during the course of this registry, we report COVID-19–related outcomes among these patients. The registry design has been previously reported,11 and additional methods of analyses are described (supplemental material).
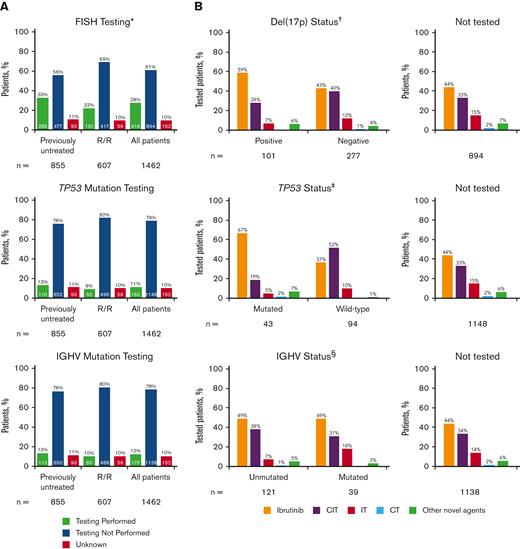
Of the 1462 eligible patients in the informCLL registry, 855 (58%) were previously untreated, and 607 (42%) had relapsed/refractory (R/R) disease. Community-based practices enrolled the majority of patients (93%). Table 1 lists the baseline demographics and clinical characteristics by LOT, and supplemental Tables 1 and 2 list these characteristics by initial treatment on the registry (index treatment). Median patient age was 71 years (range, 34-95), and most patients (88%) had an Eastern Cooperative Oncology Group performance status of 0 or 1. Consistent with previous interim analysis,11 FISH, TP53 mutation, and IGHV mutation testing were performed infrequently, occurring in less than one-third of all patients in the registry (Figure 1A). FISH testing was performed in 28% of patients (previously untreated: 33%, R/R: 22%), of whom 24% had del(17p) (previously untreated: 25%; R/R: 24%). TP53 mutation testing (11%) and IGHV mutation testing (12%) were performed in only a small fraction of patients. The large majority of patients who underwent TP53 or IGHV mutation testing also underwent other tests (supplemental Figure 1); among the patients with TP53 testing, 98% also had IGHV testing and/or FISH testing. Similarly, among those with IGHV testing, 91% also had TP53 testing and/or FISH testing. Rates of prognostic testing were similar regardless of age group (<70 years, ≥70 years) (supplemental Figure 2), insurance type (private, public, other, or none) (supplemental Figure 3), or geographic region (supplemental Figure 4), with higher rates generally seen in previously untreated than in R/R patients. FISH testing was performed more frequently in community sites than in academic sites (supplemental Figure 5).
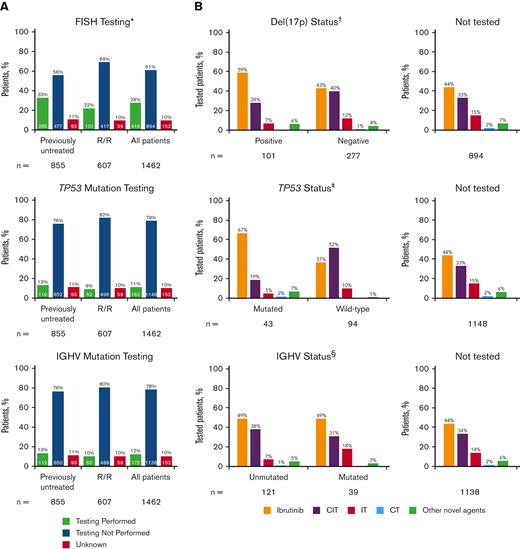
Frequency of prognostic biomarker testing by LOT and treatments received by patients in informCLL by prognostic biomarker status. (A) FISH cytogenetic testing rates, TP53 mutational status testing rates, and IGHV mutational status testing rates. (B) Treatments received by status of del(17p), TP53 mutation, and IGHV mutation. Proportions may not add up to 100% owing to rounding. ∗Includes del(17p), del(11q), del(13q), and trisomy 12. †Patients with unknown testing result (n = 38). ‡Patients with not evaluable/not specified testing result (n = 25). §Patients with not evaluable/not specified testing result (n = 12). CIT, chemoimmunotherapy; CT, chemotherapy; IT, immunotherapy.
Frequency of prognostic biomarker testing by LOT and treatments received by patients in informCLL by prognostic biomarker status. (A) FISH cytogenetic testing rates, TP53 mutational status testing rates, and IGHV mutational status testing rates. (B) Treatments received by status of del(17p), TP53 mutation, and IGHV mutation. Proportions may not add up to 100% owing to rounding. ∗Includes del(17p), del(11q), del(13q), and trisomy 12. †Patients with unknown testing result (n = 38). ‡Patients with not evaluable/not specified testing result (n = 25). §Patients with not evaluable/not specified testing result (n = 12). CIT, chemoimmunotherapy; CT, chemotherapy; IT, immunotherapy.
Ibrutinib (primarily as a single agent) was the most frequent treatment in the registry, both overall (46%) and regardless of LOT at enrollment (supplemental Table 3). CIT was the next most frequent treatment (33%; most commonly bendamustine + rituximab in 20% overall) and was more common among previously untreated (42%) than in R/R patients (20%) (supplemental Table 3). Among patients with available prognostic testing data, ibrutinib was the most common treatment for patients with high-risk genomic features, including del(17p), TP53 mutation, or unmutated IGHV (59%, 67%, and 49%, respectively) (Figure 1B). Approximately one-fifth to one-third of the patients who had del(17p), TP53 mutation, or unmutated IGHV received CIT (28%, 19%, and 38%, respectively). A large proportion of patients in this registry did not undergo FISH (72%), TP53 mutation (89%), or IGHV mutation (88%) testing or did not have testing results available, yet CIT was administered in approximately one-third of these patients.
In previously untreated patients, use of ibrutinib increased over the course of enrollment, with corresponding decrease in the use of CIT and IT over time (supplemental Figure 6). In R/R patients, use of ibrutinib remained largely consistent over time, with approximately half of the patients receiving ibrutinib as index treatment, whereas use of other novel agents increased over time. Conversely, use of CIT, IT, and chemotherapy tended to decrease in the R/R population. Treatment selection over time was further evaluated by age group (<70 years, ≥70 years), comorbidity burden (CCI <3, CCI ≥3), high-risk genomic features (del(17p)/TP53 mutation, unmutated IGHV), and institution type (community sites, academic sites) (supplemental Figures 7-11). Over time, an increase in treatment with ibrutinib and a decrease in the use of CIT were more pronounced among patients with del(17p)/TP53 mutation, particularly among the previously untreated group; however, use of first-line CIT persisted (∼20% in 2019) among these high-risk patients (supplemental Figure 9). Use of ibrutinib was more frequent across LOT among patients treated at academic centers, with more persistent use of CIT and IT at community sites (supplemental Figure 11).
As of 30 July 2021, COVID-19 infection with positive laboratory test results was reported in 50 of 1055 patients (5%) who remained on the registry as of 17 February 2020 (start date of the COVID-19 observation period); the incidence rate of COVID-19 was 3.7 per 1000 patient-months. Among the 50 patients with reported COVID-19 infection, the median age was 67 years (range, 43-86 years) based on time of registry enrollment. Among these, 24 patients were receiving first-line treatment at registry enrollment, whereas the remaining 26 were receiving second-line or greater treatment at enrollment; 16 patients had received ≥2 regimens before enrollment. The median CCI score at time of enrollment was 1 (range, 0-4) with a score of 0 or 1 in 70%, score of 2 in 22%, and score of >2 in 8%. The most common comorbidities included hypertension (60%), arthropathies (22%), diabetes (20%), chronic obstructive pulmonary disease/pulmonary issues (16%), thyroid disorders (14%), and cardiac arrhythmias (14%). At the time of COVID-19 diagnosis, 31 patients were actively receiving CLL therapy (or were within 30 days from last dose of CLL treatment regimen); these regimens included ibrutinib (n = 19), acalabrutinib (n = 2), venetoclax-based regimens (n = 7), and rituximab (n = 3).
Among the 50 patients, COVID-19–related adverse events (AEs) were reported in 46, with hospitalization due to COVID-19 in 28; COVID-19–related AEs resolved in 31 patients. Overall, 14 out of 50 (28%) of patients with reported COVID-19 infection had a fatal outcome. This case fatality rate appears similar to the 33% fatality rate reported from a multicenter, international cohort study in patients with CLL diagnosed with symptomatic COVID-19 during the earlier part of the pandemic.12 Relative to patients who had resolution of COVID-19–related AEs, patients with fatal outcomes with COVID-19 infection tended to be older (median age 74 vs 64 years), were more heavily pretreated (43% vs 23% with ≥2 lines of previous CLL therapy before registry enrollment), had a history of hypertension (71% vs 55%), and had a history of smoking (64% vs 39% were current or former smokers).
In conclusion, results from informCLL showed that although the selection of CLL treatment has shifted over time, one-third of patients continued to receive CIT, including those with del(17p)/TP53 mutation, a high-risk population known to experience poor outcomes with CIT.13-15 Despite treatment guideline recommendations,8,16 more than two-thirds of patients from the registry did not have appropriate prognostic testing performed at enrollment and may have potentially received suboptimal therapies. These findings highlight the need for greater awareness of current guideline recommendations and the importance of prognostic testing to optimize treatment decisions.
Acknowledgments: The authors thank the patients who participated in the study, their supportive families, and the investigators and clinical research staff from the study centers.
Editorial support was provided by Ying Hou, of PharmaWrite, and Agnieszka Looney, of ApotheCom, and was funded by Pharmacyclics LLC, an AbbVie Company and Janssen.
This study was cosponsored by Pharmacyclics LLC, an AbbVie Company, and Janssen.
Contribution: A.R.M., N.G., J.P.S., D.B., M.G., and J.C.B. provided study material or patients; S.U., A.Y., and M.N. provided collection and assembly of data; and all authors had access to the data, contributed to the conception and design of the study, contributed to the analysis and interpretation of data, manuscript writing, and final approval of the manuscript, and are accountable for all aspects of the work.
Conflict-of-interest disclosure: A.R.M. has served in a consultancy/advisory role with AbbVie, Acerta, Adaptive, AstraZeneca, BeiGene, DTRM Biopharma, Genentech, Curio, Dava, Octopharma, Janssen, Johnson and Johnson, LOXO, Nurix, Genmab, BMS, Pharmacyclics LLC, an AbbVie Company, Sunesis, and TG Therapeutics; has received research funding from AbbVie, Octopharma, Acerta, Adaptive, BeiGene, DTRM Biopharma, Genentech, Genmab, Nurix, Janssen, Johnson and Johnson, LOXO, Pharmacyclics LLC (an AbbVie Company), Sunesis, and TG Therapeutics; and has had other relationship(s) with TG Therapeutics (DSMB). N.G. has served in a consultancy/advisory role with AbbVie, Adaptive Biotech, ADC Therapeutics, BeiGene, Bristol Myers Squibb, Genmab, Gilead/Kite, Incyte, Janssen, Pharmacyclics LLC, an AbbVie Company, and TG Therapeutics; has received research funding from Bristol Myers Squibb, Genentech, Pharmacyclics LLC, an AbbVie Company, and TG Therapeutics; and has served on a speakers' bureau for AbbVie, Bristol Myers Squibb, Epizyme, Gilead/Kite, Janssen, and Pharmacyclics LLC, an AbbVie Company. J.P.S. has received honoraria from AbbVie, ADC Therapeutics, AstraZeneca, BeiGene, Genentech, Lilly, Pharmacyclics LLC, an AbbVie Company, and TG Therapeutics and has served in a consultancy/advisory role with AbbVie, AstraZeneca, and BeiGene. D.B. has served in a consultancy/advisory role with AbbVie, ArQule/Merck, Genentech, Pfizer, Pharmacyclics LLC, an AbbVie Company, and TG Therapeutics; has received research funding paid to institution from AbbVie, ArQule, Ascentage, AstraZeneca, BeiGene, DTRM, Genentech, Juno Therapeutics/Celgene/Bristol Myers Squibb, CATO SMS, NeWave, MEI Pharma, Novartis, Pharmacyclics LLC, an AbbVie Company, and TG Therapeutics; and has other relationship(s) with NCCN (panel member), AbbVie (informCLL registry steering committee), and Pfizer (Biosimilars outcomes research panel). M.N. has been employed by Pharmacyclics LLC, an AbbVie Company and has stock ownership in AbbVie. A.Y. has been employed by Pharmacyclics LLC, an AbbVie Company, during conduct of the study and has stock ownership in AbbVie. S.U. has been employed by Pharmacyclics LLC, an AbbVie Company; has stock ownership in AbbVie, has a family member who is employed by and owns stock in Protagonist Therapeutics, and has a patent with Biomea Fusion. Q.H. has been employed by Janssen Scientific Affairs, LLC and owns stock in Johnson & Johnson. L.H.W. has been employed by Janssen Biotech, Inc, has patents, royalties, and other intellectual property with Janssen Biotech, Inc, and owns stock in Janssen Biotech, Inc. J.C.B. has received honoraria from Janssen, has served in a consultancy/advisory role with AbbVie, AstraZeneca, BeiGene, and MEI Pharma, and has received research funding from Oncternal Therapeutics, Pharmacyclics LLC, an AbbVie Company, and VelosBio. M.G. declares no competing financial interests.
Correspondence: Jacqueline Barrientos, Mount Sinai Comprehensive Cancer Center, 4306 Alton Rd, 3rd Floor, Miami Beach, FL 33140; e-mail: jacqueline.barrientos@msmc.com.
References
Author notes
Requests for access to individual participant data from clinical studies conducted by Pharmacyclics LLC, an AbbVie Company, can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
The full-text version of this article contains a data supplement.