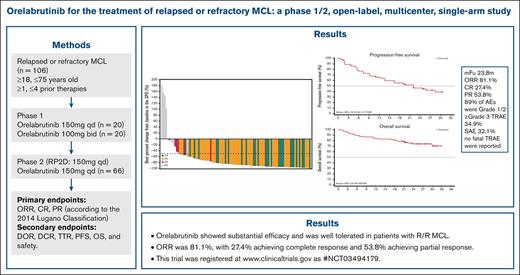
Key Points
Orelabrutinib exhibited a favorable efficacy and safety profile in patients with r/r MCL.
Overall response rate was 81.1%, with 27.4% achieving complete response and 53.8% achieving partial response.
Abstract
Relapsed or refractory (r/r) mantle cell lymphoma (MCL) is an aggressive B-cell malignancy with a poor prognosis. Bruton tyrosine kinase (BTK) is a mediator of B-cell receptor signaling and is associated with the development of B-cell lymphomas. Patients with r/r MCL were enrolled in this phase 1/2 study and treated with orelabrutinib, a novel, highly selective BTK inhibitor. The median number of prior regimens was 2 (range, 1-4). The median age was 62 years (range, 37-73 years). Eligible patients received oral orelabrutinib 150 mg once daily (n = 86) or 100 mg twice daily (n = 20) until disease progression or unacceptable toxicity. A dose of 150 mg once daily was chosen as the preferred recommended phase 2 dose. After a median follow-up duration of 23.8 months, the overall response rate was 81.1%, with 27.4% achieving a complete response and 53.8% achieving a partial response. The median duration of response and progression-free survival were 22.9 and 22.0 months, respectively. The median overall survival (OS) was not reached, and the rate of OS at 24 months was 74.3%. Adverse events (AEs) occurring in >20% of patients were thrombocytopenia (34.0%), upper respiratory tract infection (27.4%), and neutropenia (24.5%). Grade ≥3 AEs were infrequent and most commonly included thrombocytopenia (13.2%), neutropenia (8.5%), and anemia (7.5%). Three patients discontinued treatment because of treatment-related adverse events (TRAEs), but no fatal TRAEs were reported. Orelabrutinib showed substantial efficacy and was well tolerated in patients with r/r MCL. This trial was registered at www.clinicaltrials.gov as #NCT03494179.
Introduction
Mantle cell lymphoma (MCL) is a subtype of B-cell non-Hodgkin lymphoma that results from the malignant transformation of B lymphocytes in the mantle zone of lymph node follicles. MCL is a rare hematologic malignancy with an incidence rate of ∼0.8 per 100 000 people in the United States.1 MCL occurs most frequently in men at a median age of 60 years, and most patients are in an advanced stage of disease when diagnosed.2,3 Despite high response rates after first-line chemoimmunotherapy, most patients relapse and require subsequent treatment.4 There is no standard therapy for relapsed/refractory (r/r) MCL, and the therapies approved by the US Food and Drug Administration for this patient population, such as bortezomib or lenalidomide monotherapy, are still limited, with low rates of complete response (CR), short durations of remission, and unfavorable safety and tolerability for older patients.
Bruton tyrosine kinase (BTK) inhibitors represent a new class of disease-targeting agents shown to promote apoptosis of malignant B cells and reduce their proliferation and maturation, resulting in high antitumor activity.5-9 Ibrutinib is the first-in-class BTK inhibitor and has shown significant activity in a pivotal phase 2 study in patients with r/r MCL, in which a response rate of 65.8% was observed.10 Ibrutinib is associated with notable side effects, including diarrhea, infection, atrial fibrillation, and bleeding events.10,11 Although efficacious, clinical use of ibrutinib in patients with r/r MCL is compromised because of high rates of discontinuation (9%) and dose reductions (14%) due to these adverse events (AEs).10 Acalabrutinib and zanubrutinib are second-generation BTK inhibitors approved under accelerated approval for the treatment of r/r MCL. These agents have improved safety towing to less off-target toxicity relative to ibrutinib.12,13 However, high incidences of AEs such as diarrhea, atrial fibrillation, and hemorrhage are still concerns for these drugs associated with off-target activities.14,15
Orelabrutinib is a novel, highly selective, irreversible BTK inhibitor with high target selectivity and safety. Orelabrutinib was approved in China in December 2020 for the treatment of adult patients with r/r MCL who have received at least 1 prior therapy. Findings from in vitro studies showed that orelabrutinib has high potency and exceptional kinase selectivity for BTK (half-maximal 50% inhibitory concentration: 1.6 nM), with notable less off-target inhibition of other tyrosine kinases.16,17 In a kinase screening assay against a panel of 456 kinases, orelabrutinib at a concentration of 1 μM inhibited BTK to a significant level (>90%) and had minimal off-target binding.16,17 Compared with other BTK inhibitors, orelabrutinib exhibited a high selectivity profile.18 Orelabrutinib exhibited linear pharmacokinetic characteristics with dose-proportional increases in plasma exposure.16,17 Orelabrutinib also demonstrated high BTK target occupancy in peripheral blood of healthy subjects, which was maintained at close to 100% over a 24-hour interval at doses of ≥50 mg/d.16,17 The high selectivity and persistent occupancy of the BTK target are expected to lead to substantial improvements in safety and efficacy. Based on these results, we conducted a phase 1/2 multicenter, open-label clinical trial investigating the safety and efficacy of orelabrutinib in patients with r/r MCL.
Methods
Patients
Male and female patients were ≥18 and ≤75 years old with histopathological confirmed MCL (documented as t [11;14]-positive and/or with high expression of cyclin D1) and had ≥1 and ≤4 prior therapies and relapsed or failed to achieve response. Other eligibility criteria included Eastern Cooperative Oncology Group performance status score ranging from 0 to 2 and adequate hematologic status (neutrophil count ≥1.5 × 109/L and platelet count ≥75 × 109/L), renal function (serum creatinine ≤1.5 × upper limit of normal), and hepatic function (total bilirubin ≤2 × upper limit of normal and transaminases ≤2.5 × upper limit of normal).
Study design and treatment
This multicenter, open-label phase 1/2 study was divided into 2 stages. In the first stage, patients with r/r MCL were randomly assigned to receive orelabrutinib at 100 mg twice daily (n = 20) or 150 mg once daily (n = 20). Based on the pharmacokinetics results, BTK occupancy in peripheral blood mononuclear cells (PBMCs), safety, tolerability, and preliminary efficacy, the recommended phase 2 dose (RP2D) was determined to be 150 mg once daily. In the second stage, additional patients with r/r MCL (n = 66) were enrolled at the 150 mg once daily dose level. Orelabrutinib was administered orally in 28-day cycles until progression of disease or intolerable toxicity. Blood samples were collected from 17 patients in cycle 1 on day 1 and day 15 (predose and up to 12 hours post-dose), day 2 and day 16 (predose, 150 mg once daily cohort only), and day 28 (predose) to evaluate the pharmacokinetic of orelabrutinib. Blood samples were also collected for evaluation of BTK receptor occupancy (pharmacodynamic marker). Orelabrutinib plasma concentrations were analyzed via high-performance liquid chromatography with tandem mass spectrometry, and pharmacokinetic parameters were estimated via noncompartmental analysis using Phoenix WinNonlin (version 7.0). A systematically validated enzyme-linked immunosorbent assay was used to determine BTK occupancy in PBMC lysate for pharmacodynamic data analysis.
All patients provided written informed consent. Full details of the study methodology can be found from the attached protocol.
Patient evaluation
Initial assessment included a physical examination, standard laboratory assessments, computed tomography (CT)/magnetic resonance imaging (MRI) of the neck, chest, abdomen, and pelvis, and a bone marrow biopsy. Tumor evaluation will be performed during the screening period, every 8 weeks in cycles from 1 to 12, and every 12 weeks in cycle 13 and afterwards. Imaging results (enhanced CT, MRI, plain CT, etc) within 28 days before enrollment were acceptable. If bone marrow was involved at baseline, clinical suspicion of CR through a physical examination or CT required confirmation via a bone marrow examination, including immunohistochemistry examination, within 4 weeks. Follow-up assessments included routine blood counts, serum immunoglobulin and β2 microglobulin level estimation, a physical examination, and a 12-lead electrocardiogram and echocardiogram. Response was evaluated per the 2014 Lugano Classification.19
Outcomes
The primary end point was the overall response rate (ORR), defined as the proportion of CR and partial response (PR), assessed by an independent review committee (IRC) in accordance with the 2014 Lugano Classification.19 Secondary end points included ORR assessed by investigators, duration of response (DOR), disease control rate, time to response (TTR), progression-free survival (PFS), overall survival (OS), and safety. The severity of AEs was graded in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03).20
Assessments of the pharmacokinetics and pharmacodynamics of orelabrutinib were secondary end points. Pharmacokinetic parameters to be evaluated included area under the curve from zero to the last time point (AUC0-t), area under the curve from zero to infinity (AUC0-∞), maximum concentration (Cmax), time to maximum concentration, elimination half-life (t1/2), clearance, volume of distribution, and accumulation factor. Samples for pharmacodynamic analysis were sent to a central laboratory for isolation of PBMCs to measure the BTK target occupancy of orelabrutinib.
Results
Patients and treatment
A total of 106 patients with r/r MCL were enrolled and received orelabrutinib, with 40 in the first stage and 66 in the second stage. Of the 106 enrolled patients in the full analysis set, 79.2% were male, with a median age of 62 years (range, 37-73 years), and 30 patients (28.3%) were ≥65 years. One hundred patients (94.3%) were in an advanced stage III or IV disease, and 43 patients (40.6%) had bone marrow infiltration. Forty-two patients (39.6%) had intermediate- or high-risk MCL international prognostic index scores. Bulky disease (longest transverse diameter of a lesion) >10 cm and >5 cm was found in 10.4% and 28.3% of patients, respectively. The median sum of the products of diameters (SPD) of lymphoma lesions was 2514.4 mm2. The median number of prior lines of therapy was 2 (range, 1-4). Thirty-two (30.2%) patients had refractory disease. Detailed demographic and baseline characteristics of the enrolled patients are presented in Table 1. The median time on study was 23.8 months, and patients received treatment for a median of 21.1 months (range, 7.4-27.4 months). By the cut-off date of 31 December 2020, 54 patients had discontinued study treatment. The reasons for treatment discontinuation included disease progression (43), patient request (5), AEs (3), death (1), investigator decision (1), and other (1; patient used chemotherapy outside hospital).
Pharmacokinetics/pharmacodynamics and RP2D selection
The pharmacokinetic and pharmacodynamic characteristics of orelabrutinib were evaluated in 17 patients with r/r MCL (n = 8 at 100 mg twice daily and n = 9 at 150 mg once daily). Orelabrutinib was rapidly absorbed after a single oral dose of 100 mg twice daily or 150 mg once daily, and the median time to maximum concentration values were essentially identical at 1.9 and 2.0 hours, respectively (supplemental Figure 1). Average t1/2 values after single-dose administration of 150 mg once daily or 100 mg twice daily were 4.1 and 2.5 hours, respectively. This discrepancy in t1/2 may be due to differences in sampling time points during the terminal elimination phase. Orelabrutinib exhibited linear pharmacokinetic characteristics, and systemic exposure (Cmax and AUC) increased proportionally with dose increase. There was no apparent drug accumulation observed and no significant changes in pharmacokinetic parameters after multiple dosing for 15 days, and pharmacokinetic behavior remained unchanged. Coefficients of variation of pharmacokinetic parameters were <30%, indicating that individual pharmacokinetic differences of orelabrutinib in patients with r/r MCL were small (supplemental Table 1). Because orelabrutinib is an irreversible BTK inhibitor, its efficacy is expected to be primarily driven by Cmax. Compared with 100 mg twice daily, 150 mg once daily with a higher Cmax is expected to achieve higher target engagement and higher efficacy.
BTK occupancy by orelabrutinib was used as a pharmacodynamic biomarker of BTK inhibition. Both 100 mg twice daily and 150 mg once daily of orelabrutinib achieved close to 100.0% BTK occupancy, and this effect was sustained up to 24 hours in the PBMCs of patients with MCL. The interindividual variability between patients was small (supplemental Figure 2).
As of 1 August 2018, a total of 14 patients completed at least 1 investigator-led imaging assessment, including 8 patients in the 150 mg once daily group and 6 patients in the 100 mg twice daily group. The efficacy (ORR) of the 2 dose groups met the target objective (65%), but that of the 150 mg once daily dose group was slightly superior (supplemental Table 2). Most AEs were grades 1 or 2. Treatment-emergent AE and treatment-related adverse events are summarized in the supplement (supplemental Table 3). Two AEs in the 100 mg twice daily dose group led to drug interruption. There were no treatment-emergent AEs or treatment-related adverse events leading to treatment discontinuation. No serious AEs (SAEs) occurred in both dose groups. From a safety perspective, orelabrutinib was well tolerated in both dose groups of 100 mg twice daily and 150 mg once daily.
Based on pharmacokinetic/pharmacodynamic profile, clinical efficacy, and safety data in the first stage, 150 mg once daily was selected to evaluate orelabrutinib monotherapy in the second stage, given that a once daily dosing regimen has better compliance in clinical practice.
Treatment outcomes
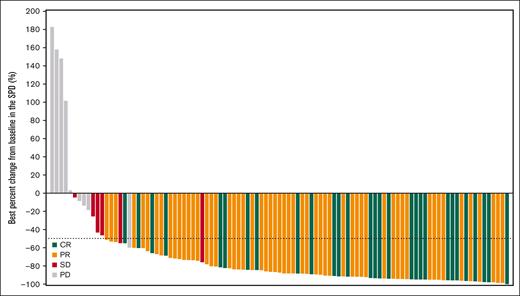
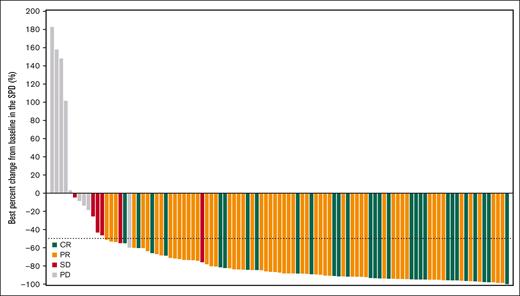
In the 106 patients who received orelabrutinib, the median follow-up was 23.8 months. Table 2 shows the investigator- and IRC-assessed responses. Via IRC assessment, objective response was recorded in 86 patients (81.1%; 95% confidence interval [CI], 72.4-88.1), with 27.4% of patients achieving CR and 53.8% of patients achieving PR. Six patients (5.7%) were assessed as having stable disease, and the disease control rate was 86.8%. Via investigator assessment, 87 patients (82.1%) had an objective response, with 34.9% of patients achieving CR and 47.2% of patients achieving PR. The concordance rate between IRC- and investigator-assessed responses was 93.4%. The IRC-assessed CR rate improved over time from 25.5% at 10.5-month follow-up to 27.4% at 23.8-month follow-up (supplemental Table 4). CT/MRI results showed that a reduction in lymphadenopathy was observed in all but 5 patients, and the median reduction percentage in the SPD of target lesions was 87.2% from a baseline median SPD of 2514.4 mm2 in most of all 106 treated patients (Figure 1).
Best percentage change from baseline in the SPD of target lesions for all 106 patients treated with orelabrutinib.
Best percentage change from baseline in the SPD of target lesions for all 106 patients treated with orelabrutinib.
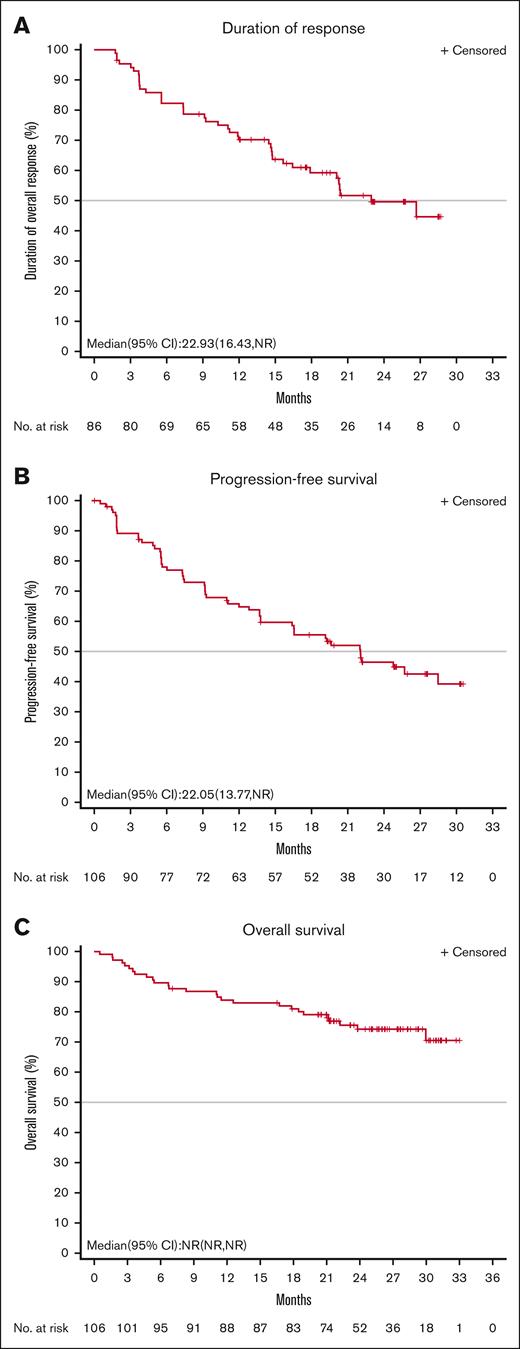
Patients achieved a rapid response. The median TTR was 1.9 months. At the data cut-off date, the median DOR was 22.9 months (95% CI, 16.4 to not reached), and the proportion of subjects with a DOR ≥ 24 months was 49.6% (Figure 2A). The median PFS was 22.0 months (95% CI, 13.8 to not reached), and the PFS rate at 24 months was 46.5% (Figure 2B). The median OS was not reached, and the rate of OS at 24 months was 74.3% (95% CI, 64.4-81.8; Figure 2C). Among the 29 patients who achieved a best overall response of CR, the DOR and PFS rates at 24 months neared 80%, and the OS at 24 months remained near 100% (Figure 3).
Duration of response, progression-free survival, and overall survival in full analysis set population. (A) IRC-assessed DOR, (B) PFS, and (C) overall survival in full analysis set population. NR, not reached.
Duration of response, progression-free survival, and overall survival in full analysis set population. (A) IRC-assessed DOR, (B) PFS, and (C) overall survival in full analysis set population. NR, not reached.
Duration of response, progression-free survival, and overall survival in patients achieving CR, PR, or who were non-responders (SD/PD). (A) IRC-assessed DOR, (B) PFS, and (C) overall survival.
Duration of response, progression-free survival, and overall survival in patients achieving CR, PR, or who were non-responders (SD/PD). (A) IRC-assessed DOR, (B) PFS, and (C) overall survival.
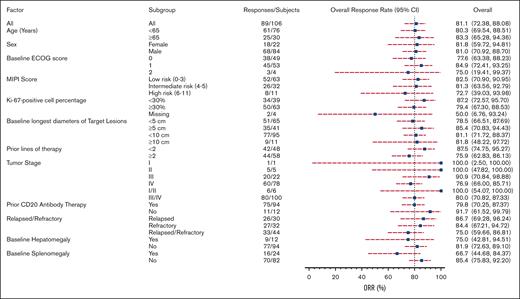
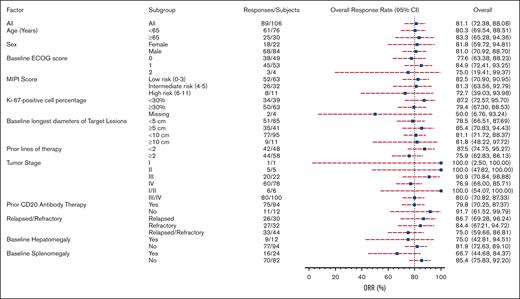
Response to orelabrutinib was consistent across subgroups, with treatment benefits observed in all prespecified subgroup analyses. Overall response (assessed by the IRC) was observed in 83.3% of elderly patients, 80% with stage III/IV disease, 81.8% with bulky disease, 72.2% with high-risk MCL international prognostic index scores, and 79.4% with Ki-67 levels ≥30 (Figure 4). Notably, efficacy was improved in patients who had not received prior treatment with anti-CD20 monoclonal antibodies. Among the 12 patients who were not exposed to anti-CD20–based therapy, the ORR was 91.7%. However, a baseline splenomegaly was associated with a lower rate of response at 66.7%.
Subgroup analysis of ORR assessed by the IRC based on baseline demographic and clinical characteristics.
Subgroup analysis of ORR assessed by the IRC based on baseline demographic and clinical characteristics.
Safety
The safety profile was evaluated for a total of 106 patients who received orelabrutinib treatment, among whom 73 patients (68.9%) received orelabrutinib for more than 1 year. The median duration of exposure was 21.1 months (interquartile range, 7.4-27.4), and the median relative dose intensity was 99.6%. Most AEs (89%) observed with orelabrutinib treatment were grade 1 or 2. Among all 106 patients, the most common AEs of any grade occurring in more than 10% of patients were thrombocytopenia (34.0%), upper respiratory tract infection (27.4%), neutropenia (24.5%), leukopenia (19.8%), anemia (19.8%), pneumonitis (17.9%), hypokalemia (17.0%), rash (17.0%), hypertension (17.0%), cough (16.0%), hyperglycemia (12.3%), pyrexia (12.3%), weight increase (11.3%), prolonged QT interval (11.3%), pneumonia (11.3%), diarrhea (11.3%), alanine aminotransferase increase (10.4%), hematuria (10.4%), and hypoalbuminemia (10.4%) (Table 3).
Grade 3 or higher AEs that were reported in >2 patients included thrombocytopenia (13.2%), neutropenia (8.5%), anemia (7.5%), pneumonia (5.7%), hypertension (5.7%), disease progression (4.7%), lymphocytosis (3.8%), leukocytosis (3.8%), pneumonitis (3.8%), soft tissue infection (2.8%), and hypokalemia (2.8%) (Table 3). Grade 3 or higher AEs were considered treatment-related in 37 patients (34.9%). SAEs occurred in 34 patients (32.1%), and the SAEs reported in ≥2 patients were thrombocytopenia (6.6%), pneumonia (5.7%), progressive disease (4.7%), pneumonitis (4.7%), femoral neck fracture (1.9%), and anemia (1.9%) (supplemental Table 5).
AEs leading to discontinuation of treatment occurred in 5 patients (4.7%), of whom 3 patients (2.8%) discontinued treatment because of drug-related AEs: 2 cases of thrombocytopenia and 1 case of hemoptysis. Seven patients (6.6%) had AEs leading to dose reduction, all of which were treatment-related, and 28 patients (26.4%) had AEs leading to dose interruption, of which 18 cases (17%) were determined to be treatment-related. A total of 27 patients died during the study, including 16 deaths (15%) from disease progression, 2 deaths (2%) from AEs deemed unrelated to treatment, 1 death (1%) from lung infection due to the common cold, 1 death (1%) from infection due to acute myeloid leukemia, and 7 deaths (7%) of unknown cause. No deaths due to drug-related AEs were reported.
Infection, cytopenia, second primary malignancy, diarrhea, hypertension, bleeding, and atrial fibrillation are the most concerning AEs in clinical treatment with BTK inhibitors. Infection and cytopenia were relatively common AEs reported in this study. Infection events considered to be drug-related occurred in 34% of patients, of which upper respiratory tract infection (13.2%) and pneumonia (7.5%) were the most common. Grade 3 or higher infection events occurred in 14.2% of patients, and 6.6% were deemed drug-related. Treatment-related cytopenia, including anemia and decreases in platelets, neutrophils, and white blood cells, occurred in 14.2%, 32.1%, 22.6%, and 18.9% of patients, respectively. Other notable AEs were uncommon. Three second primary malignancy events (acute myeloid leukemia, squamous cell carcinoma of lung, and bladder cancer) were observed but assessed as unrelated to orelabrutinib. One patient with squamous cell carcinoma presented with lung lesions at screening, and this second malignancy was not considered a new occurrence. Diarrhea was reported in 11.3% of patients, all of which were grade 1 or 2, with 3.8% judged as related to treatment. Hypertension occurred in 17% of patients and was evaluated as treatment-related in 15.1% of patients. Thirty-seven patients (34.9%) experienced bleeding events, all of which were grade 1 (33 patients, 31.1%) or grade 2 (4 patients, 3.8%) and most commonly included hematuria (10.4%), microscopic hematuria (6.6%), petechiae (4.7%), and purpura (4.7%). No patient experienced major hemorrhage (defined as grade 3 or higher, serious, or any grade central nervous system bleeding event). Only 1 episode of grade 1 atrial fibrillation was reported in cycle 1 that did not recur and was deemed unrelated to treatment. No grade 3 or higher second primary malignancy, atrial fibrillation/flutter, diarrhea, or bleeding events were observed.
Discussion
Orelabrutinib is a novel, irreversible BTK inhibitor with high potency and superior target selectivity compared with previously known BTK inhibitors. Daily dosing of 150 mg once daily resulted in continuous and nearly 100% BTK occupancy over 24 hours in patients with r/r MCL, which surpassed the >90% occupancy rate observed with ibrutinib at doses ≥175 mg/d.10 The sustained and complete BTK occupancy of orelabrutinib in PBMCs is presumed to induce the potent and durable responses observed in this study. Based on the results of pharmacokinetics, BTK inhibition in PBMCs, safety, tolerability, preliminary efficacy, and compliance for patients, 150 mg once daily was chosen as the preferred RP2D. After a median follow-up of 23.8 months, single-agent orelabrutinib induced a high ORR of 81.1% and CR of 27.4% by IRC and an ORR of 82.1% and CR of 34.9% by the investigator as assessed by CT/MRI. The median DOR was 22.9 months, and the median PFS was 22 months. The demographic and baseline disease characteristics of patients in this study were comparable to those in studies conducted with ibrutinib, acalabrutinib, and zanubrutinib in terms of sex, age, disease stage, bone marrow infiltration, Eastern Cooperative Oncology Group performance status, tumor lesion size, and median prior lines of therapy.12,21,22 In the ibrutinib pivotal study 1104 in patients with r/r MCL, ibrutinib showed an ORR of 65.8% and CR of 17.1% after a median 15.3-month follow-up with CT-based tumor assessment. The median DOR and PFS were 17.5 months and 13.9 months, respectively.10,21 The effect of acalabrutinib and zanubrutinib were also investigated in r/r MCL based on positron emission tomography (PET) assessment, with ORRs of 81% (CR, 43%) and 84% (CR, 68.6%) after a median follow-up of 26 months and 18.4 months, respectively. Median DOR and PFS were 26 months and 20 months for acalabrutinib and 19.5 months and 22.1 months for zanubrutinib, respectively.22,23 It is noteworthy that unlike this study and the ibrutinib study 1104 that used CT-based tumor assessment, PET-CT imaging was used in the acalabrutinib and zanubrutinib trials, which showed higher CR rates. PET is highly sensitive to metabolic activities for assessment in lymphomas, whereas CT can only reveal the size of the tumor.24,25 Nonetheless, the PFS and OS of zanubrutinib, acalabrutinib, and orelabrutinib were comparable, all of which were higher than the PFS and OS of ibrutinib.
Such a high rate of response observed with orelabrutinib was consistent across prespecified subgroups, including older patients aged ≥65 years (83.3%), patients with bulky disease (81.8%), and patients with ≥2 prior lines of therapy (75.9%). Compared with ibrutinib at a median follow-up of 26.7 months, orelabrutinib demonstrated a superior ORR in patients with bulky tumors (81.8% vs 67%) and ≥2 prior treatments (75.9% vs 63%).11 Moreover, with extended treatment time, depth of remission improved and the proportion of subjects who achieved CR also increased. CR patients had significantly higher DOR and PFS than those who achieved PR or had no response. These high and durable responses in DOR and PFS also translated into clinically meaningful survival benefits, with nearly 100% of complete responders surviving at ≥24 months (Figure 3).
It is noteworthy that rapid reductions of extramedullary disease were observed along with a median TTR (1.9 months). Of interest, the median TTRs were the same for ibrutinib (1.9 months)21 and 2.8 months for zanubrutinib.26
The safety and tolerability of orelabrutinib in patients with r/r MCL were favorable. The rate of treatment discontinuation in this study (4.7%) was low relative to that in ibrutinib (9%) and zanubrutinib (12.5%) studies.10,13 The median duration of exposure and relative dose intensities were high. The most common AEs were grades 1 or 2 and did not require additional treatment. Events of cytopenia and infections (including upper respiratory infection and pneumonia) were expected as they are commonly reported from the same class of drugs and were mostly mild to moderate. Diarrhea occurred in 11.3% of patients in this study, with no reports of grade 3 or higher severity. This is a meaningful difference compared with incidences of diarrhea with ibrutinib (43%),10 acalabrutinib (31%),14 and zanubrutinib (23%).15 In addition, only 1 case of grade 1 atrial fibrillation was observed with orelabrutinib, whereas other BTK inhibitors have reported grade 3 or greater atrial fibrillation and atrial flutter in ∼1.1% to 4% of patients, with ibrutinib having the highest incidence at 4%.10,14,15 A second primary malignancy was reported in 3 patients treated with orelabrutinib; however, evidence of lesions was present at screening in 1 case of squamous cell carcinoma of lung. The rate of second malignancy in this study (2.8%) was significantly lower than incidences observed with ibrutinib (10%),10 acalabrutinib (12%),14 and zanubrutinib (14%).15 Major hemorrhage has also been reported in 4%, 5.4%, and 3.0% of patients treated with ibrutinib, zanubrutinib, and acalabrutinib,10,13,14 respectively, whereas no cases of major hemorrhage were observed with orelabrutinib therapy. In addition, lower rates of grade 3 or higher infection were observed with orelabrutinib than with other BTK inhibitors (14.2% vs 19%-27%).10,14,15 The incidence of hypertension in this study (17%) was comparable to rates reported with ibrutinib (19%), but grade 3 or higher hypertension events were lower (5.7% vs 8%).10
As a single-arm study, the sample size is limited in this study and there is no active control arm. A larger randomized study is needed to further confirm the efficacy and safety of orelabrutinib in patients with MCL. In addition, PET imaging was not used in this study for metabolic assessment of tumor response; therefore, the CR rate based on CT imaging only may be underestimated in this study.27
Orelabrutinib has shown potent activity in patients with r/r MCL and substantial improvement in response rates compared with ibrutinib. In addition, orelabrutinib exhibited a favorable safety profile with a lower incidence of AEs observed in the BTK inhibitor class, supporting a safe and highly effective treatment option for patients with r/r MCL. The further development of orelabrutinib is expected to provide additional evidence of its clinical benefit and risk profile compared with available therapies.
Acknowledgments
The authors thank all patients who participated in this study and their families as well as all the investigators and site staff who made the study possible.
This study was sponsored by InnoCare Pharma Limited.
Authorship
Contribution: X.-Y.Z., R.-B.Z., B.Z., and Y.-M.T. designed the study; L.-J.D., K.-S.Z., L.-H.L., M.-Z.Z., Z.-M.L., C.-Y.J., W.X., T.L., B.X., X.W., S.-J.G., H.-L.Z., Y.H., Y.L., Y.C., H.-Y.Y., J.-N.C., Z.-M.Z., J.-D.H., W.Z., H.-M.J., K.-Y.D., Y.-P.S., Y.-Q.S., and J.Z. were principal investigators and collected the data; B.Z. and Y.-M.T. analyzed the data and were responsible for manuscript development and editing; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: X.-Y.Z., R.-B.Z., B.Z., and Y.-M.T. are employees of InnoCare Pharma Limited. The remaining authors declare no competing financial interests.
Correspondence: Jun Zhu, Department of Lymphoma, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Peking University Cancer Hospital & Institute, 52 Fucheng Rd, Haidian District, Beijing 100142, China; e-mail: zhu-jun2017@outlook.com.
References
Author notes
Data are available on request from the corresponding author, Jun Zhu (zhu-jun2017@outlook.com).
The full-text version of this article contains a data supplement.