Key Points
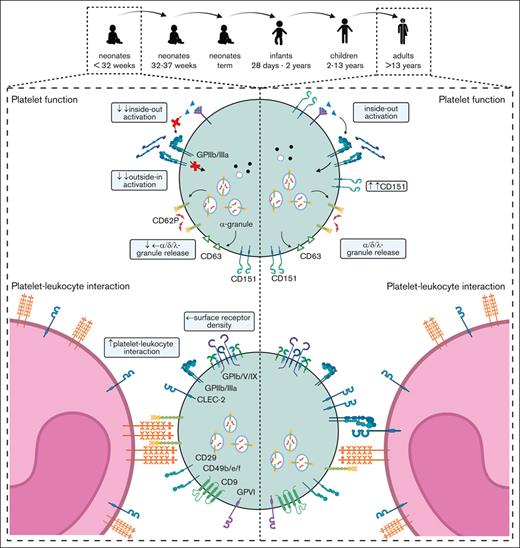
The platelet subpopulation pattern evolves continuously from the neonatal period until adulthood, especially GPIIb/IIIa responsiveness.
Agonist-induced integrin GPIIb/IIIa activation and granule release are uncoupled in neonates.
Abstract
Erythrocytes undergo a well-defined switch from fetal to postnatal circulation, which is mainly reflected by the stage-specific expression of hemoglobin chains. Perinatal alterations in thrombopoiesis are poorly understood. We assessed the ontogenesis of platelet phenotype and function from early prematurity to adulthood. We recruited 64 subjects comprising 7 extremely preterm (27-31 weeks gestational age), 25 moderately preterm (32-36 weeks), 10 term neonates, 8 infants (<2 years), 5 children (2-13 years), and 9 adults (>13 years). Blood was withdrawn at up to 3 different time points in neonates (t1: 0-2, t2: 3-7, and t3: 8-14 days after birth). We found that the expression levels of the major surface receptors for fibrinogen, collagen, vWF, fibronectin, and laminin were reduced but correlated with decreased platelet size, indicating a normal surface density. Although CD62P and CD63 surface exposure upon stimulation with TRAP-6, ADP, or U46619 was unaltered or only slightly reduced in neonates, GPIIb/IIIa inside-out and outside-in activation was blunted but showed a continuous increase until adulthood, correlating with the expression of the GPIIb/IIIa regulating tetraspanin CD151. Platelet subpopulation analysis using automated clustering revealed that neonates presented with a CD63+/PAC-1– pattern, followed by a continuous increase in CD63+/PAC-1+ platelets until adulthood. Our findings revealed that the number of platelet-monocyte and platelet-neutrophil aggregates, but not platelet-lymphocyte aggregates, is increased in neonates and that neonatal aggregate formation depends in part on CD62P activation. Our PLatelets In Neonatal Infants Study (PLINIUS) provides several lines of evidence that the platelet phenotype and function evolve continuously from neonates to adulthood.
Introduction
Platelets are the second most abundant cells in circulation and are best known as key mediators of primary hemostasis. Over the last few decades, they have additionally been recognized as important regulators of the immune defense1 and vascular integrity.2 During embryogenesis, platelets contribute to the separation of the blood and lymphatic vessels, and a lack of platelets around birth leads to delayed patent ductus arteriosus closure.3,4 These distinct platelet functions before birth might be reflected by the profound differences between fetal and adult thrombopoiesis.5 Developmental differences in erythrocyte development are well recognized, mostly due to hemoglobin gene switches during embryogenesis and in the adults.6
Although erythrocytes have a lifespan of 120 days, it is only ∼8 days in platelets, so they become continuously replenished by their precursor cells, designated as megakaryocytes (MK). In adults, bone marrow (BM) MKs release platelets into the vessels. However, in developing organisms, platelets are mainly derived from the fetal liver. Data from mice and humans have shown that there are fundamental differences between fetal liver and BM-derived MKs, reflected in different platelet functions.7 Platelets in neonates are reported to be “hyporeactive” when compared with adults. Several studies in preterm neonates have concluded that platelet function to agonists such as ADP, thrombin, or collagen is even more impaired (reviewed in Andres et al8). Some studies on platelet function have used umbilical cord-derived platelets, which are known to differ in protein expression from venous blood platelets. These (pre)analytical issues add an additional layer of complexity to the characterization and understanding of neonatal platelet biogenesis and functions.9
Recently, differences between adult and neonatal platelet function have become evident in a seminal clinical study, reporting that transfusion of count-adjusted (adult) platelets from concentrates into neonates below 34 weeks of gestational age (GA) at lower blood platelet thresholds (<25 cells/nL) led to a decreased bleeding risk and mortality compared with higher blood platelet thresholds (<50 cells/nL).10 Understanding the ontogenesis of platelet function from embryofetogenesis during the perinatal period into the first months of life is of immediate clinical interest, not only to improve pediatric platelet transfusion regimens or to counteract the increased bleeding risk, such as intraventricular hemorrhage in preterm neonate,11 but also to expand our knowledge on fetal and neonatal platelet function and how this changes over time. Because the ontogenesis of platelets from fetal to postnatal circulation is still incompletely understood, we aimed to prospectively assess the ontogenesis of platelet phenotype and function from the earliest feasible time point at 28 weeks of GA until adulthood in a cross-sectional study. Hence, we recruited 66 participants comprising 44 preterm and term neonates. We found that the density of major platelet surface receptors was unaltered. Intriguingly, GPIIb/IIIa responsiveness evolved slowly, but continuously until adulthood. This was reflected by the gradually evolving pattern of platelet subpopulations. In summary, our PLatelets In Neonatal Infants Study (PLINIUS) provides experimental evidence that platelet biogenesis and function evolves gradually from fetal hematopoiesis into adulthood as an overall continuous developmental process.
Materials and methods
Study cohort and sample generation
The study was approved by the ethical committee of the University of Würzburg (EV 92/13) according to the Declaration of Helsinki. Participants were recruited consecutively over 2 years within the neonatal intensive care unit and wards of the Department of Pediatrics at the University Hospital Würzburg. Informed parental consent was obtained before inclusion in the study. Venous blood was withdrawn from the scalp, saphenous, or superficial dorsal vein in sodium citrate monovettes (3.2%) within defined time intervals, as indicated (supplemental Figure 1). The exclusion criteria for infants, children, and adults comprised any syndromic disorder, intrauterine transfusions/surgery or transfusion within the last 4 weeks, hemato-oncological disease, coagulopathy, or self-reported nonsteroidal anti-inflammatory drug (NSAID) intake within the last 2 weeks.
Statistical analysis
Statistical analysis was performed using GraphPad Prism Version 9.2. (San Diego, CA). Differences between groups were analyzed using the Kruskal-Wallis test. Correlation was assessed by calculating Spearman r. A correlation value of r > 0.5 was considered strong and values between 0.3 and 0.5 as intermediate. The outlier testing was performed using the ROUT test.
Further methods are included in the supplemental Material.
Results
Patient cohort
We recruited a convenience sample of preterm and term neonates from the earliest feasible time point after birth and prospectively followed these infants for 2 further assessments in the first weeks of life. To compare age-specific receptor expression and function, we stratified our cohort of 64 participants into 6 groups: (I) extremely preterm neonates (<32 weeks GA), (II) moderately preterm neonates (32-36 weeks GA), (III) term neonates (37-41 weeks GA), (IV) infants (28 days [d]-2 years [a]), (V) children (2a-13a), and (VI) adults (>13a). Bleeding occurred in 1 preterm infant (cohort II, grade 2 intraventricular hemorrhage) and 1 child (cohort V, IgA vasculitis). None of the infants had proven bacterial infection or sepsis. Two children received ibuprofen for patent ductus arteriosus (therapy start: 3/4 days after birth). Blood withdrawal was scheduled at birth (t1: 0-2 days) and for neonates (subcohorts I-III) at up to 2 follow-up time points (t2: 3-7 days, t3: 8-14 days) to monitor development after birth. 21 of the 42 neonates were born by spontaneous vaginal delivery and 21 of the 42 by cesarean delivery (6 by nonelective cesarean delivery, 1 by vacuum extraction, and no use of forceps). There was 1 case of maternal HELLP syndrome and 4 cases of preeclampsia. The mothers of 15 neonates received general anesthesia, 14 received regional anesthesia, and 13 did not received anesthesia. In total, 77 blood samples were analyzed, including 24 follow-up measurements (Table 1; supplemental Figure 1).
Unaltered receptor density on neonatal platelets
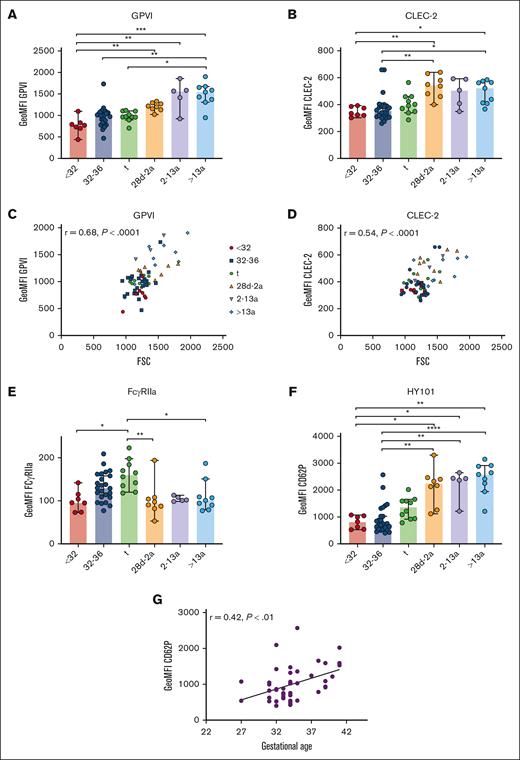
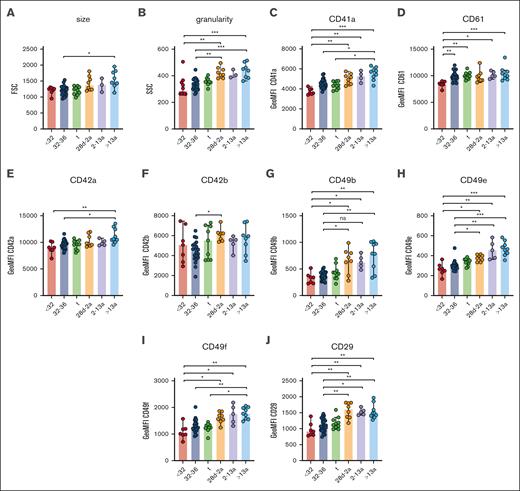
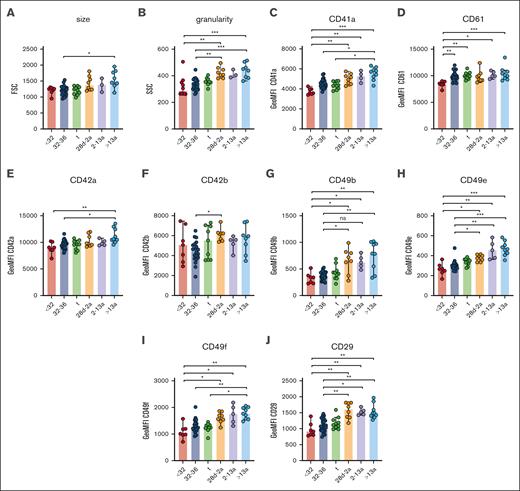
Neonatal thrombocytopenia is a frequent finding in neonates, especially in preterm infants.12 In our cohort, 40 of 42 neonates presented with platelet counts within their age-dependent reference range, whereas only 2 neonates were thrombocytopenic (124 cells/nL, GA: 33 + 6 weeks; 132 cells/nL, 34 + 0 weeks). The median platelet count in infants (28d-2a) was 245 cells/nL in children (2a-13a) 344 cells/nL and in adults 272 cells/nL (Table 1), which reflects a regularly observed age-dependent decrease in platelet count.13 Platelet size and granularity enlarged with GA, as shown by higher values for forward- (FSC) and side-scatter (SSC) in our flow cytometric analysis (Figure 1A-B), implying that platelets are somewhat larger (FSC) and thus might contain more granules (SSC). The expression levels of receptors for fibrinogen (CD41/CD61) (Figure 1C-D), von Willebrand factor (vWF) (CD42a/CD42b) (Figure 1E-F), collagen (α2; CD49b) (Figure 1G), fibronectin (α5; CD49e) (Figure 1H), laminin (α6; CD49f) (Figure 1I), and their common integrin β1 subunit (CD29) (Figure 1J) increased also in an age-dependent manner. The latter values were strongly correlated with platelet size and granularity, indicating an overall unaltered receptor density on the platelet surface in early preterm neonates (supplemental Figure 3).
Unaltered receptor density in neonatal platelets. Characteristics of preterm neonates <32 weeks GA, 32 to 36 weeks GA, >36 weeks GA (t), infants 28d-2a, children 2 to 13a and adults >13a are displayed. (A) Forward- (FSC) and (B) side-scatter (SSC), (C) CD41a, (D) CD61, (E) CD42a, (F) CD42b, (G) CD49b, (H) CD49e, (I) CD49f, and (J) CD29 expression were assessed by flow cytometry in whole blood. All graphs show the median ± 95% confidence interval values. Differences were analyzed using the Kruskal-Wallis test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. ns, nonsignificant.
Unaltered receptor density in neonatal platelets. Characteristics of preterm neonates <32 weeks GA, 32 to 36 weeks GA, >36 weeks GA (t), infants 28d-2a, children 2 to 13a and adults >13a are displayed. (A) Forward- (FSC) and (B) side-scatter (SSC), (C) CD41a, (D) CD61, (E) CD42a, (F) CD42b, (G) CD49b, (H) CD49e, (I) CD49f, and (J) CD29 expression were assessed by flow cytometry in whole blood. All graphs show the median ± 95% confidence interval values. Differences were analyzed using the Kruskal-Wallis test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. ns, nonsignificant.
Different mechanisms of platelet preactivation in neonates and adults
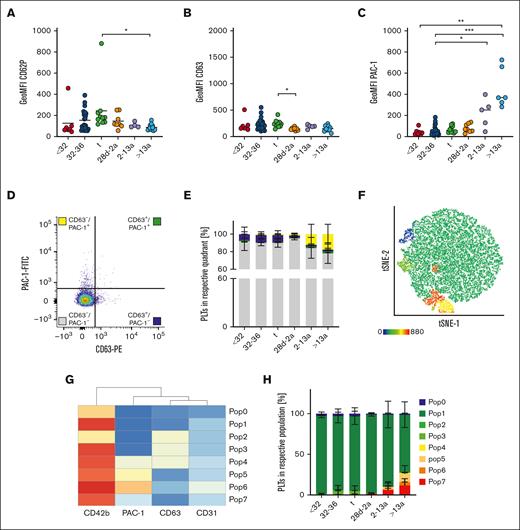
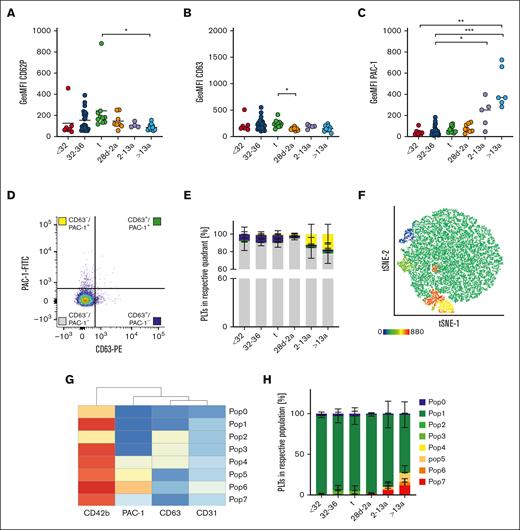
Platelet activation results mainly in integrin GPIIb/IIIa activation and the release of internally stored granules. CD62P is a marker for α-granule release, and CD63 for δ- and lysosomal (λ)-granule release. We assessed these markers under resting conditions to check for platelet preactivation in the first blood sample available from each neonate or proband (t1). CD62P geometric mean fluorescence intensity (GeoMFI) values were <200 in infants, children, and adults (Figure 2A). The values appeared to be elevated in some neonates, and we identified 3 outliers using the ROUT test by applying a sensitive parameter of Q = 10%. This increase was not associated with needle size or complications during blood withdrawal. CD63 exposition was also low (Figure 2B). We detected a higher degree of GPIIb/IIIa activation in platelets of adults (GeoMFI resting: 404 vs ADP: 3488) (Figure 2C), although the degree was far below those values found in response to agonists so that these platelets can readily be considered as “resting” rather than “preactivated.”14 To analyze granule release and integrin activation concomitantly on a single cell level, we used resting (adult) platelets to set 2% threshold gates for both, CD63 surface exposure together with GPIIb/IIIa activation (PAC-1 binding). The majority of events throughout all age cohorts under resting conditions were CD63–/PAC-1– (Figure 2E, gray bars). Approximately 7% to 8% of platelets in all 3 neonate subcohorts were CD63+/PAC-1– (blue bars), whereas this subpopulation was absent in infants, children, and adults. In adults (cohort VI), we found that more platelets were CD63–/PAC-1+ than in the neonatal cohorts (I-III) (yellow bars). The gate setting for quadrant analysis is not well standardized and partly arbitrary. Thus, we performed an unbiased clustering approach in a subset of subjects (n = 29) to corroborate our findings of distinct platelet subpopulations. In addition to the 2 activation markers, CD63 and PAC-1, we included CD42b as part of the GPIb/V/IX complex, which can be cleaved in response to agonists, and CD31, a marker constitutively expressed on platelets. Further, most platelets were CD63–/PAC-1– double negative, but we detected a few more PAC-1+ single positive platelets in the adult cohort (6, Pop6: 4%) (Figure 2F-H). Taken together, our findings imply that from extremely preterm neonates to adults, the vast majority of platelets are in a resting state and do not express CD63 or GPIIb/IIIa activation.
Different mechanisms of platelet preactivation in neonates and adults. Characteristics of preterm neonates <32 weeks GA, 32 to 36 weeks GA, >36 weeks GA (t), infants 28d-2a, children 2 to 13a and adults >13a are displayed. (A) CD62P, (B) CD63 expression, and (C) GPIIb/IIIa activation were assessed by flow cytometry on resting platelets in whole blood. (D,E) Quadrant analysis of CD63 and PAC-1 was performed. (F-H) Automated clustering analysis of resting platelets. (G) Heat map of platelet subpopulations. Representative curves are shown in panels D and F. The median ± 95% confidence interval (CI) values are displayed in panels A-C. The mean ± 95% CI is shown in panels E and H. Differences were analyzed using the Kruskal-Wallis test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. ns, nonsignificant.
Different mechanisms of platelet preactivation in neonates and adults. Characteristics of preterm neonates <32 weeks GA, 32 to 36 weeks GA, >36 weeks GA (t), infants 28d-2a, children 2 to 13a and adults >13a are displayed. (A) CD62P, (B) CD63 expression, and (C) GPIIb/IIIa activation were assessed by flow cytometry on resting platelets in whole blood. (D,E) Quadrant analysis of CD63 and PAC-1 was performed. (F-H) Automated clustering analysis of resting platelets. (G) Heat map of platelet subpopulations. Representative curves are shown in panels D and F. The median ± 95% confidence interval (CI) values are displayed in panels A-C. The mean ± 95% CI is shown in panels E and H. Differences were analyzed using the Kruskal-Wallis test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. ns, nonsignificant.
Uncoupling of α- and δ- granule release from GPIIb/IIIa activation in neonates
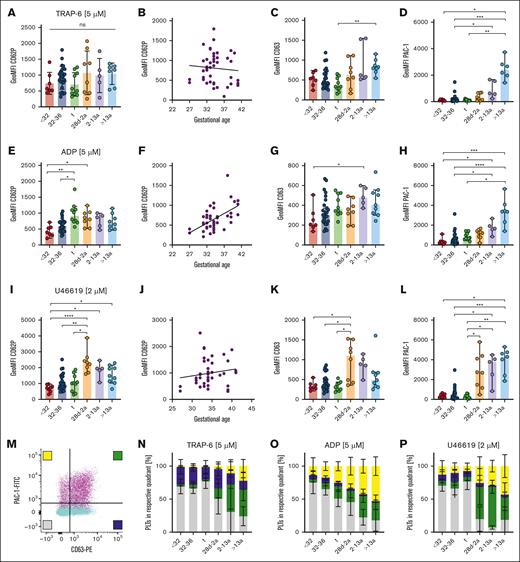
Next, we assessed platelet activation using suboptimal and an optimal dose of agonists TRAP-6, ADP, and U46619. Activation with high doses (5 μM) of the thrombin receptor agonist TRAP-6 induced CD62P and CD63 exposure on the platelet surface in preterm (cohorts I, II) and term (III) neonates. CD62P GeoMFI levels in these cohorts (I) 739, (II) 914, and (III) 705) were overall comparable with the levels detected in (IV) infants (1069), (V) children (985), or (VI) adults (1040), indicating distinct functional α- and δ/λ-granule release independent of the GA with a lack of direct correlation (Figure 3A-C; supplemental Figure 4A-C). In contrast, platelets derived from all 3 neonate cohorts (I-III) and infants (IV) were less capable of activating GPIIb/IIIa upon TRAP-6 stimulation when compared with platelets of children (V) or adults (VI) (Figure 3D; supplemental Figure 4D). Incubation with high (5 μM) or low-dose ADP (2 μM) led to less exposure of CD62P or CD63 in neonatal subcohorts (I-III), but CD62P values correlated with GA (r = 0.55; P < .001) (Figure 3E-G; supplemental Figure 4E-G). We observed again a reduced GPIIb/IIIa activation in neonates (I-III) and infants (IV) than in children (V) and adults (VI) (Figure 3H; supplemental Figure 4H), implying that integrin activation upon ADP stimulation is muted until adulthood. The TxA2 receptor agonist U46619 phenocopied our findings of functional α- and δ/λ-granule release and diminished GPIIb/IIIa activation for ADP in all neonates (I-III), both in high (2 μM) and low (0.5 μM) concentrations (Figure 3I-L; supplemental Figure 4I-L).
Uncoupling of α- and δ- granule release from GPIIb/IIIa activation in neonates. Characteristics of preterm neonates <32 weeks GA, 32 to 36 weeks GA, >36 weeks GA (t), infants 28d-2a, children 2 to 13a and adults >13a are displayed. Whole blood was preincubated with (A-D) TRAP-6 (5 μM), (E-H) ADP (5 μM), or (I-L) U46619 (2 μM). (A,B,E,F,I,J) CD62P or (C,G,K) CD63 expression or (D,H,L) GPIIb/IIIa activation were assessed flow cytometry. (M-P) Quadrant analysis of CD63 and PAC-1 upon (M,N) TRAP-6, (O) ADP, or (P) U46619 stimulation was performed. Representative curves are shown in panel M. The median ± 95% confidence interval (CI) is displayed in panels A-L. Mean ± 95% CI is shown in panels N-T. Differences were analyzed using the Kruskal-Wallis test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ns, nonsignificant.
Uncoupling of α- and δ- granule release from GPIIb/IIIa activation in neonates. Characteristics of preterm neonates <32 weeks GA, 32 to 36 weeks GA, >36 weeks GA (t), infants 28d-2a, children 2 to 13a and adults >13a are displayed. Whole blood was preincubated with (A-D) TRAP-6 (5 μM), (E-H) ADP (5 μM), or (I-L) U46619 (2 μM). (A,B,E,F,I,J) CD62P or (C,G,K) CD63 expression or (D,H,L) GPIIb/IIIa activation were assessed flow cytometry. (M-P) Quadrant analysis of CD63 and PAC-1 upon (M,N) TRAP-6, (O) ADP, or (P) U46619 stimulation was performed. Representative curves are shown in panel M. The median ± 95% confidence interval (CI) is displayed in panels A-L. Mean ± 95% CI is shown in panels N-T. Differences were analyzed using the Kruskal-Wallis test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ns, nonsignificant.
Some agonists led to a slight increase in reactivity in follow-up samples (from t1 to t2), as previously reported.15,16 The reduced platelet responsiveness compared with that in adults might thus be intrinsic to GA rather than the birth itself (supplemental Figure 5). In line with this, we did not find an association between reduced platelet function and mode of birth (vaginal delivery vs cesarean delivery) or maternal anesthesia (general vs regional vs no anesthesia) (supplemental Figure 6).
As integrin activation and granule release typically occur concomitantly in response to platelet stimulation, we sought to address whether these 2 mechanisms of activation might be uncoupled in neonatal platelets, which would be reflected by an increased subpopulation of platelets positive for CD63 and/or CD62P, but negative for integrin activation (PAC-1). Platelets were stimulated with TRAP-6, ADP, or U46619, and quadrant analysis was repeated using the “2% over threshold” gates for CD63 and PAC-1, as shown for resting platelets (Figure 2D). TRAP-6 treatment revealed an unexpected clear separation of neonatal platelet populations (I-III) compared with that in adults (VI) (Figure 3M-N; supplemental Figure 7A). For the latter, we found the expected combination of integrin activation and granule release, reflected by the high degree of double-positive events (green bars). However, neonates (I-III) presented a CD63+/PAC-1– biased response (indicated by blue bars in Figure 3N), with the virtual absence of CD63–/PAC-1+ (yellow bars) or double-positive events. This uncoupling of granule release from integrin activation was also present upon stimulation with ADP (Figure 3O; supplemental Figure 7B) or U46619 (Figure 3P; supplemental Figure 7C). Analysis of blood samples from infants (IV) and children (V) revealed a steady increase in CD63–/PAC-1+ (yellow bars) or double-positive events, suggesting the gradual development of GPIIb/IIIa responsiveness to various agonists over time. Notably, we also evaluated the release of α-granules (CD62P) with integrin activation in a subset of patients. The results virtually phenocopy our findings for CD63 (supplemental Figure 8A-C), which was further corroborated by the strong correlation of CD62P neo-exposition with CD63 after stimulation with ADP, TRAP-6, and U46619 throughout our entire neonatal cohort (supplemental Figure 8D-F), providing additional evidence that the release of α- as well as δ/λ-granules occurs concomitantly.
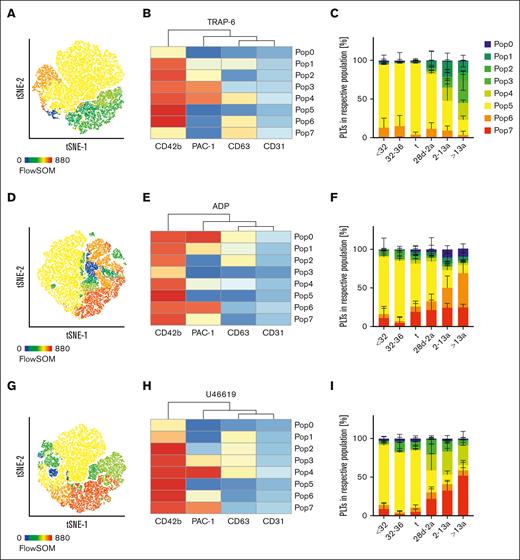
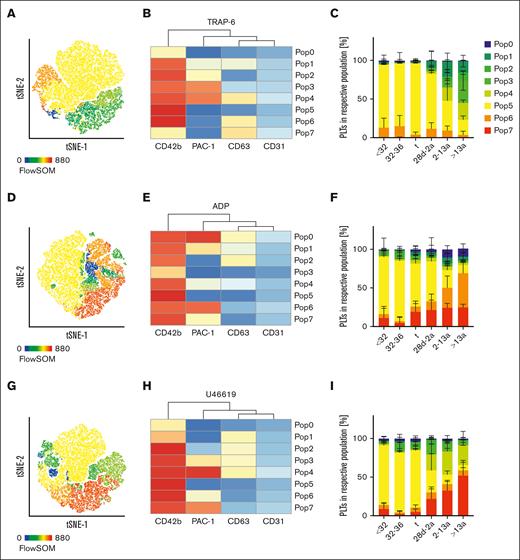
Recent single cell transcriptional analyses on MKs revealed the existence of different subpopulations that might eventually result in platelet subpopulations with different functions.17 This is supported by the presence of distinct platelet populations in circulation with a bias toward either CD62P exposure or GPIIb/IIIa integrin activation, depending on the type of agonist.18 Our previous findings from the quadrant analysis suggested that the slowly shifting pattern of platelet subpopulations might evolve gradually from neonates until adulthood. Thus, we performed an automated clustering analysis using FlowSOM and depicted the results using a t-distributed stochastic neighbor embedding (t-SNE) dimension reduction approach. In response to TRAP-6, we found that the number of GPIIb/IIIa expressing subpopulations increased continuously from preterm neonates into adulthood (Figure 4A-C; supplemental Figure 7A). In neonatal cohorts (I-III), <1% of all events were PAC-1high (Pop3/4); however, in adults, 51% of the platelets were PAC-1high. The intermediate PAC-1+ subpopulation (Pop2) was <1% in all neonates (I-III), but increased continuously in children (IV:3%), infants (V:6%), and adults (VI:10%). In contrast, CD63+ single-positive platelets were predominantly found in preterm neonates (I:13%; II:15%), whereas they were virtually absent in adults (VI:4%). Similar findings were observed in response to ADP (Figure 4D-F) and U46619 (Figure 4G-I). Taken together, our data provide evidence for the continuous evolution of platelet subpopulations during neonatal development from infants to adults, especially for GPIIb/IIIa responsiveness.
Maturation of platelet subpopulations. Characteristics of preterm neonates <32 weeks GA, 32 to 36 weeks GA, >36 weeks GA (t), infants 28d-2a, children 2 to 13a and adults >13a are displayed. Whole blood was preincubated with (A-C) TRAP-6 (5 μM), (D-F) ADP (5 μM), or (G-I) U46619 (2 μM). Automated clustering analysis was performed using FlowSOM. Representative curves are shown in panels A,D,G. Heat maps of clustering are shown in panels B,E, and H.
Maturation of platelet subpopulations. Characteristics of preterm neonates <32 weeks GA, 32 to 36 weeks GA, >36 weeks GA (t), infants 28d-2a, children 2 to 13a and adults >13a are displayed. Whole blood was preincubated with (A-C) TRAP-6 (5 μM), (D-F) ADP (5 μM), or (G-I) U46619 (2 μM). Automated clustering analysis was performed using FlowSOM. Representative curves are shown in panels A,D,G. Heat maps of clustering are shown in panels B,E, and H.
GPIIb/IIIa can also sense substrates such as fibrinogen and mediate an “outside-in” signaling cascade, which is reflected by shape change and platelet spreading.19 We assessed this “outside-in” signaling in washed platelets plated on fibrinogen-coated coverslips. Owing to limited biomaterial and a comparable pattern of responsiveness among our neonatal cohorts, we restricted this analysis to term neonates compared with adults. After platelet adhesion to fibrinogen for 45 minutes, we found significantly more platelets in stage 1 (resting platelet adhesion) and stage 2 (filopodia formation) in neonates than in adults. Consequently, fewer platelets in neonates were able to form lamellipodia (stage 3) or fully spread (stage 4) (Figure 5A-B). In summary, we provide evidence that the GPIIb/IIIa outside-in response develops gradually from neonate to adulthood.
Reduced CD151 expression in neonates. Characteristics of preterm neonates <32 weeks GA, 32 to 36 weeks GA, >36 weeks GA (t), infants 28d-2a, children 2 to 13a, and adults >13a are displayed. (A) Representative images and (B) quantification of the different spreading phases of fixed term neonate and control platelets 45 minutes after differential interference contrast imaging (Zeiss Axiovert 200 inverted microscope [100×/1.4 oil objective]). (C-D) CD9 and (E-F) CD151 expression were assessed in whole blood using flow cytometry. (D) CD9 expression vs forward scatter (FSC) (F) PAC-1 binding after ADP (5 μM) stimulation vs CD151 expression. All graphs show the median ± 95% confidence interval. Differences were analyzed using Kruskal-Wallis-test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ns, nonsignificant.
Reduced CD151 expression in neonates. Characteristics of preterm neonates <32 weeks GA, 32 to 36 weeks GA, >36 weeks GA (t), infants 28d-2a, children 2 to 13a, and adults >13a are displayed. (A) Representative images and (B) quantification of the different spreading phases of fixed term neonate and control platelets 45 minutes after differential interference contrast imaging (Zeiss Axiovert 200 inverted microscope [100×/1.4 oil objective]). (C-D) CD9 and (E-F) CD151 expression were assessed in whole blood using flow cytometry. (D) CD9 expression vs forward scatter (FSC) (F) PAC-1 binding after ADP (5 μM) stimulation vs CD151 expression. All graphs show the median ± 95% confidence interval. Differences were analyzed using Kruskal-Wallis-test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ns, nonsignificant.
GPIIb/IIIa is highly expressed on the platelet surface and its distribution within the lipid bilayer is partly organized by tetraspanins such as CD9 or CD151.20 CD9 expression was unaffected by overall age and FSC (r = −0.15; P = .26) (Figure 5C-D). The expression of CD151, which was reported to be crucial for GPIIb/IIIa activation,21 was markedly reduced in neonatal platelets (I-III: GeoMFI: 639) and infants (IV:577) with an age-dependent increase (V:1195; VI:1941) (Figure 5E). In response to agonists, the expression levels correlated with PAC-1 binding (r = 0.60; P < .0001) (Figure 5F), implying that CD151 could be a potential mediator for the development of GPIIb/IIIa-dependent platelet responsiveness.
Platelet activation induces activation of metalloproteinases ADAM10/17, inducing CD42b surface receptor shedding.22 Platelet stimulation with ADP, TRAP-6, or U46619 reduced CD42b expression in neonates (I-III) (ΔGeoMFI ADP I:2794; II:3376; III:3529) to a lesser extent than in infants, children, and adults (IV:4401; V:4965, VI:4940), suggesting the development of agonist-induced CD42b shedding (supplemental Figure 9).
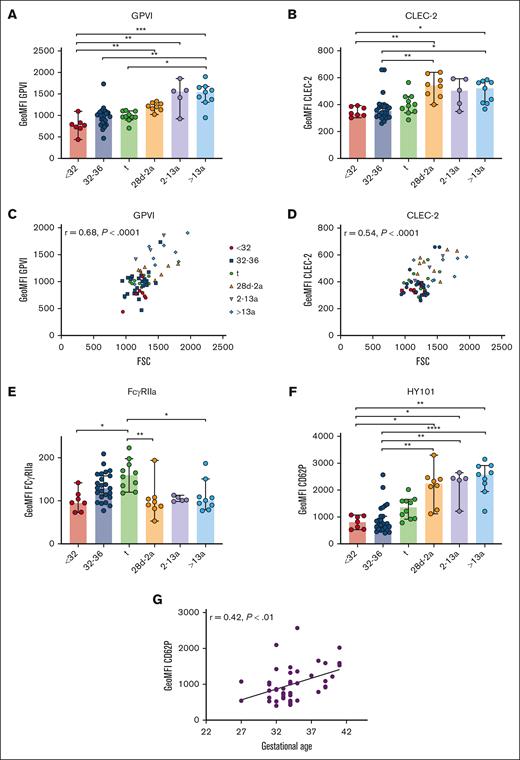
Unaltered GPVI and CLEC-2 density in neonatal platelets
Immunoreceptors are important mediators of platelet function beyond hemostasis, including the separation of blood and lymphatic vessels (C-type lectin receptor 2; CLEC-2), maintenance of vascular integrity under inflammatory conditions (GPVI), and immune defense (FcγRIIa), as shown in mice and humans.3,23-26 GPVI and CLEC-2 expression increased gradually from extremely preterm neonates (I) to adults (VI) (Figure 6A-B). The levels correlated with FSC, suggesting that receptor density remained unaltered overall during platelet maturation (CLEC-2: r = 0.54; P < .0001; GPVI: r = 0.68; P < .0001) (Figure 6C-D). Although this holds true for most other platelet receptors, there is some variation, suggesting that the overall receptor density might change only slightly during ontogenesis. Unexpectedly, FcγRIIa expression increased continuously from extremely preterm to preterm neonates, and peaked in term neonates. Infants, children, and adults had FcγRIIa surface levels comparable to those found in extremely preterm neonates (Figure 6E), inferring that the transient increase was a developmental phenomenon only during the last trimester of pregnancy.
Unaltered GPVI and CLEC-2 densities in neonatal platelets. Characteristics of preterm neonates <32 weeks GA, 32 to 36 weeks GA, >36 weeks GA (t), infants 28d-2a, children 2 to 13a and adults >13a are displayed. (A) GPVI and (B) CLEC-2 expression were assessed by flow cytometry. (C) GPVI or (D) CLEC-2 expression vs forward scatter (FSC) and (E) FcγRIIa expression are displayed. (F-G) CD62P expression after antibody (HY101) induced GPVI stimulation. (G) CD62P expression vs GA. All graphs show median ± 95% confidence interval. Differences were analyzed using Kruskal-Wallis-test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ns, nonsignificant.
Unaltered GPVI and CLEC-2 densities in neonatal platelets. Characteristics of preterm neonates <32 weeks GA, 32 to 36 weeks GA, >36 weeks GA (t), infants 28d-2a, children 2 to 13a and adults >13a are displayed. (A) GPVI and (B) CLEC-2 expression were assessed by flow cytometry. (C) GPVI or (D) CLEC-2 expression vs forward scatter (FSC) and (E) FcγRIIa expression are displayed. (F-G) CD62P expression after antibody (HY101) induced GPVI stimulation. (G) CD62P expression vs GA. All graphs show median ± 95% confidence interval. Differences were analyzed using Kruskal-Wallis-test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ns, nonsignificant.
The antibody HY101 binds to GPVI, inducing receptor dimerization and platelet activation. We observed reduced α-granule release in neonates compared with older controls (Figure 6F). CD62P exposure increased with GA exposure in the infant group (Figure 6G). Taken together, our data indicate that from extremely preterm neonates to adults, stimulation of G-protein coupled receptors (GPCRs) or immunoreceptors induces granule release that gradually increases with age.
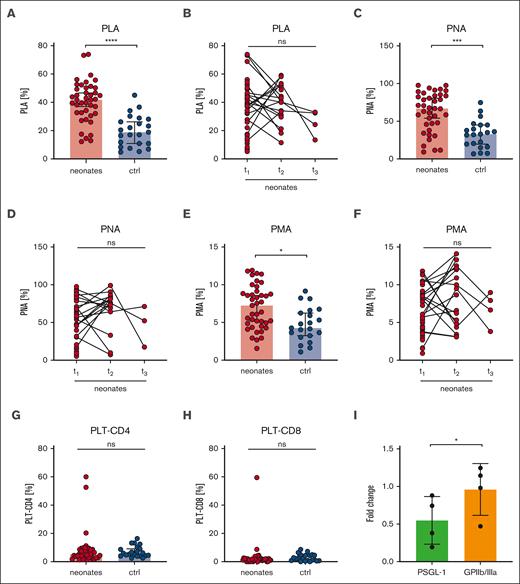
Increased platelet-leukocyte aggregates in neonates
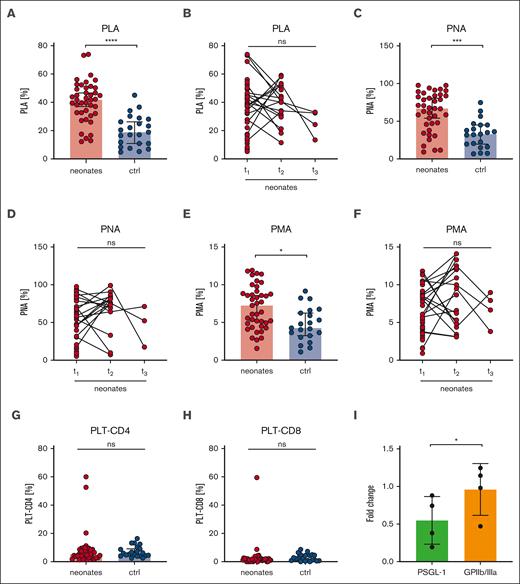
Platelets can mediate leukocyte function by direct or indirect interactions and have thus an impact on the innate immune response.27 Platelet-leukocyte interactions can be mediated by the physical binding of single or multiple platelets to leukocytes.28 We quantified platelet-leukocyte aggregates ([PLA], indicated as CD45+/CD41+ events in respect to CD45+ events in %), grouped all neonatal samples (I-III), and compared them with a combined “postbirth” group comprising infants to adults (IV-VI). Intriguingly, we found markedly increased PLA in neonates (41%) compared with that in the postbirth group (19%) (Figure 7A), which remained high until t2 (40%) (Figure 7B). The values decreased up to t3, but owing to the small sample size, no statistically significant conclusions can be drawn from this data set. PLA levels increased in preterm and term newborns independent of GA (supplemental Figure 10A). We found 62% platelet-neutrophil aggregates (PNA; indicated by CD45+/CD16+/CD41+ per CD45+/CD16+ events) compared with 32% in postbirth probands. Platelet-monocyte aggregates (PMA; CD45+/CD14+/CD41+ per CD45+/CD14+ events) were 7% in neonates compared with 5% in postbirth participants (Figure 7C-F; supplemental Figure 10B). Although PNA decreased between t2 and t3, PMA remained overall constant, suggesting that neutrophils, rather than monocytes, contributed to the dynamic decrease in PLA after birth (Figure 7D-F). Platelets are reported to undergo interactions with T cells.29 We thus assessed the degree of CD4+ and CD8+ T cells bound to platelets in the circulation but did not detect significant differences between neonates and postbirth probands (Figure 7G-H). There was no correlation between PLA or PMA and birth mode or maternal anesthesia (supplemental Figure 10C-D).
Increased platelet-leukocyte interaction in neonates. Characteristics of neonates and controls (ctrl) are displayed at t1: 0 to 2 days after birth, t2: 3 to 7 days after birth, t3: 8 to 14 days after birth. (A-B) PLA indicated as CD45+/CD41+ events in percentage of CD45+ events, (C-D) PNA indicated as CD45+/CD16+/CD41+ events in percentage CD45+/CD16+ events, (E-F) PMA indicated as CD14+/CD41+ events in percentage of CD45+, and (G) platelet-CD4+ aggregates or (H) platelet-CD8+ aggregate events were assessed by flow cytometry in whole blood. (I) Neonatal PLAs upon preincubation of whole blood with PSGL-1 blocking antibody clone KPL-1 or GPIIb/IIIa blocker eptifibatide (10 μg/ml) for 15 minutes. All graphs show median ± 95% confidence interval. Differences were analyzed using Kruskal-Wallis-test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ns, nonsignificant.
Increased platelet-leukocyte interaction in neonates. Characteristics of neonates and controls (ctrl) are displayed at t1: 0 to 2 days after birth, t2: 3 to 7 days after birth, t3: 8 to 14 days after birth. (A-B) PLA indicated as CD45+/CD41+ events in percentage of CD45+ events, (C-D) PNA indicated as CD45+/CD16+/CD41+ events in percentage CD45+/CD16+ events, (E-F) PMA indicated as CD14+/CD41+ events in percentage of CD45+, and (G) platelet-CD4+ aggregates or (H) platelet-CD8+ aggregate events were assessed by flow cytometry in whole blood. (I) Neonatal PLAs upon preincubation of whole blood with PSGL-1 blocking antibody clone KPL-1 or GPIIb/IIIa blocker eptifibatide (10 μg/ml) for 15 minutes. All graphs show median ± 95% confidence interval. Differences were analyzed using Kruskal-Wallis-test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ns, nonsignificant.
In a subgroup of neonates, we preincubated blood with the GPIIb/IIIa blocker eptifibatide or KPL-1, an antibody that blocks PSGL-1. Although KPL-1 resulted in a 50% decrease in PLA formation, eptifibatide had no effect (Figure 7I). The low CD62P levels at rest (Figure 2A) compared with agonist-triggered platelets (Figure 3A,E,I) implied that CD62P bound to leukocytes might be masked from detection. Taken together, our PLA data provide evidence that neonatal PLA formation is in part mediated by the PSGL-1/CD62P-axis rather than by GPIIb/IIIa.
Discussion
PLINIUS aimed to provide a comprehensive analysis of the ontogenesis of platelet function and phenotype from the earliest feasible time point in extremely preterm neonates until adulthood. Our novel findings are (1) Platelet surface receptor expression is overall unaltered, but platelet subpopulations evolve continuously from the neonatal period to adulthood; (2) Agonist-induced GPIIb/IIIa activation is significantly reduced in neonates, whereas granule release is only slightly affected, implying that these processes are originally separate and become coupled later during platelet ontogenesis; and (3) Platelet ontogenesis is a gradual process over time rather than a switch, as observed in red blood cells.
We exclusively used peripheral venous blood to allow direct comparison with follow-up samples and between subcohorts, which would be skewed when using umbilical blood for the initial sample. A recent study reported that umbilical blood platelets were hyperaggregable compared with peripheral blood platelets.30 Our expression approach in conjunction with the FSC provides evidence that the slightly reduced receptor expression in neonates is a direct consequence of reduced size and that platelet receptor density remained overall unaltered. When neonatal platelets were stimulated with ADP, U46619, TRAP-6, or a GPVI-activating antibody, our results not only corroborate previous findings that neonatal platelets are “hyporeactive” when compared with adult controls but also provide a comprehensive mapping of how the signaling responses change and mature over time. Overall, neonatal platelets are capable of releasing α- and δ/λ-granules, albeit not to the same extent as in adults. In contrast, GPIIb/IIIa activation was impaired and this hyporeactivity persisted into adulthood, corroborating a previous report.31 The findings from our study imply that these 2 processes are originally separate and their coupling occurs over time or are maturing in a parallel fashion. However, the use of higher agonist concentrations might partially restore GPIIb/IIIa reactivity, which would be interesting to investigate in future studies.
Our platelet subpopulation analysis in response to agonists revealed a continuous increase in PAC-1+ platelets, suggesting that this is a process of gradual development rather than a “phenotypic switch” between the platelets of neonates and adults. One unprecedented strength of our study approach is multiplex single cell analysis based on 4 distinct markers for resting and activated platelets. This allowed us to perform a complex dimensional reduction analysis, which clearly identified distinct platelet subpopulations (Pop0-Pop7). Most strikingly, the activation responses to the 3 distinct agonists reflect the gradual developmental “maturation” for each agonist separately. The response to ADP showed the most significant increase in the PAC-1+ subpopulations, which was less prominent in response to TRAP-6. This separation confirmed the agonist-specific differences shown by other groups.18 We provided experimental evidence that PLA is markedly increased in neonates, independent of their GA (supplemental Figure 10). This PLA formation was abrogated after PSGL-1 blockade. In our setup, GPIIb/IIIa blockade by the addition of eptifibatide did not affect PLA formation, as shown in previous studies,32 but other ligand-receptor pairs beyond CD62P- PSGL-1 might contribute.
To date, there have been few studies on platelet function in term and preterm neonates that combined a comprehensive set of different GAs, agonists, and readouts. In virtually all studies, platelets were considered hyporeactive toward classical agonists, such as ADP or thrombin. In addition, neonatal platelets were found to be hyperresponsive to the platelet inhibitor PGE1, also contributing to the reduced responsiveness.31,33,34,35 More recent studies have shown distinct platelet hyporeactivity upon immunoreceptor and GPCR stimulation36,37 and have discussed reduced G12/G13 expression as a causative factor for reduced δ-granule secretion.38 These controversial findings could be explained by the heterogeneous study design regarding readouts (granule release, GPIIb/IIIa activation, aggregometry, and electron microscopy), the material used (peripheral vs umbilical blood), time points, or the respective control group (adults vs infants). Comprehensive studies comprising a continuum of preterm and term neonates or numerous agonists and activation markers are still very rare, owing to limited biomaterial. Our study aimed to address these shortcomings using detailed, direct, and extended bioinformatics analyses. Because we used peripheral blood for the initial measurement, the follow-up time points were readily comparable. A recent, very elegant study by Liu et al39 used an integrated ribosome profiling/transcriptomic method to define the transition from fetal to adult platelets based on umbilical blood samples. Our study provides a complimentary set of evidence for ontogenetically divergent stages of neonatal thrombopoiesis. It remains a challenging task to bring these 2 platelet sources experimentally, considering the minute blood samples available for analysis, especially from preterm infants.
The function of distinct platelet or MK subpopulations under healthy compared with disease conditions is currently a matter of debate.40 PLINIUS has demonstrated that the complex pattern of responsiveness in distinct platelet subpopulations changes in an age-dependent manner. In neonatal circulation, the large number of hyporeactive platelets could indicate a further role of platelets beyond hemostasis, such as regulating the closure of the ductus arteriosus or supporting the immune defense, reflected by the high number of PLA.
Interestingly, not only do red blood cells and platelets show a “fetal phenotype” perinatally, but also the overall cellular immune response and the expression levels of plasmatic factors (vWF, etc) are distinct in (preterm) neonates as compared with children or adults. Thus, the hemostatic system in neonates is often considered procoagulative, probably in conjunction with less responsive platelets. A reduced GPIIb/IIIa activity could be a protective mechanism preventing disseminated thrombus formation during birth or in neonatal circulation.41 It remains enigmatic why it takes years for GPIIb/IIIa to be fully responsive. Our findings imply that neonates do not phenocopy patients with GPIIb/IIIa deficiency (Glanzmann thrombasthenia), as neonatal platelets can still aggregate in a GPIIb/IIIa-dependent manner when using higher agonist concentrations (data not shown). Notably, some studies have reported that neonates with Glanzmann thrombasthenia did not present with major bleeding symptoms after vaginal delivery,42,43 providing additional evidence that reduced GPIIb/IIIa responsiveness in neonates might reflect a physiological feature around birth.
The data from the study of Sola-Visner44 confirmed that transfusion of adult platelets into platelet-depleted neonatal whole blood resulted in markedly reduced closure time in the PFA-100 assay.45 This could partially explain why the transfusion of adult nonsilenced platelets might potentially damage the neonatal transfusion recipient, as shown in the PlaNet2 trial,10 clearly stating that “less is more.”44
The crosstalk between platelets and leukocytes enables the mutual interplay of the hemostatic system with the immune system28,46 and depends on the functional interactions of platelet surface markers with their binding partners on leukocytes.47 Previous reports have shown that the expression levels of PSGL-1 and CD62P are markedly reduced in activated fetal platelets, implying a diminished interaction between platelets and leukocytes.48,49 Unexpectedly, we observed an increased fraction of PLA, even in extremely preterm newborns, which is independent of leukocyte count, and the contributing molecules remain poorly defined.
To our knowledge, PLINIUS is the largest study to assess the ontogenesis of platelet phenotype and function. For our convenience sample, no study-specific blood withdrawal was required, but it was combined with medically indicated venipuncture. Thus, the assessed term cohort comprised 2/10 infants with slightly elevated C-reactive protein (CRP) values, but no clinical signs of infection. We evaluated several factors possibly confounding platelet function in our neonatal cohort, such as birth mode or analgesia, but did not detect any effects (supplemental Figure 6). Other factors, including intrauterine growth restriction, infection, inflammation, vascular disease, or diabetes, might also have an effect, but the analysis was not powered by this cohort size. Furthermore, we did not detect significant changes in platelet phenotype and function within our observed 2-weeks time frame. Our study had several limitations: (1) In our study, we analyzed platelets from extremely preterm neonates, moderately preterm neonates, term neonates, infants, and children recruited in a hospital setup according to our inclusion/exclusion criteria (supplemental Figure 1). Preterm delivery (cohorts I/II) occurs in a somewhat pathological situation, whose underlying reasons may remain elusive in some cases. Patients in cohorts III-V were hospitalized, implying that they could not be classified as fully healthy. Nevertheless, we consider that the findings presented in this study are overall valid and reflect coherent platelet ontogenesis. (2) The investigation of extremely preterm neonatal infants faces multiple challenges that we could not address in this study design. Although we could exclude that some major confounders, including birth mode or maternal anesthesia conditions, had a major effect on platelet function (supplemental Figure 6), further studies are needed to address how these factors impact neonatal hemostasis and platelet function. (3) Our study focused on neonatal platelet function in response to platelet agonists. Platelet inhibitors were not included in the panel because of the more complex and overall limited readouts. However, some studies have provided evidence that platelets are hyperresponsive to inhibiting agents, including prostaglandin PGE1.34 In addition, altered expression of G protein subunits Gα12/13 and G αi2 might interfere with the responsiveness to classical agonists such as collagen or thrombin, leading to an increased basal cAMP level in neonatal platelets, which might contribute to a “hyporesponsive” platelet phenotype.38 The diminished response to TRAP-6 might also be a consequence of the reduced expression; a future study might elaborate on thrombin responsiveness with more concentrations and including a PAR4 peptide. (4) Because of the limited blood samples available from preterm and term neonates, we decided to select concentrations according to the current (inter)national guidelines. However, the concentrations used in this study may be “optimal” or saturated for (preterm) neonates. We demonstrated that aggregation could be induced when higher doses of agonists (ie, CRP-XL) were used (data not shown). The evaluation of dose-response curves requires further investigations. PAC-1 can be replaced by fluorophore-conjugated fibrinogen, implying that our results, based on antibody binding, reflect the expected functional properties of activated GPIIb/IIIa (supplemental Figure 11). (5) Owing to ethical reasons, the number of follow-up samples (t3) in neonates was limited.
In summary, PLINIUS provides experimental evidence that platelets undergo a continuous trajectory of functional and phenotypic development, in contrast to the overall rapid postnatal phenotypic switch between fetal and adult erythrocytes. Understanding these gradual changes in distinct activation cascades will require future investigations to further decipher platelet ontogenesis.
Acknowledgments
The authors thank Gaby Haase for providing the technical and experimental support. L.J.W. was funded in part by Deutsche Forschungsgemeinschaft (German Research Foundation), grants 374031971-TR 240 and 453989101-SFB 1525. M.D. was funded by IZKF grant Z-02/84.
Authorship
Contribution: L.J.W. performed experiments and data analysis, provided intellectual input, designed figures and wrote the manuscript; M.D. and K.M. provided intellectual input and performed data analysis; S.B., D.U., and B.J. performed experiments and data analysis; C.H. and C.P.S. provided intellectual input and financed parts of the study; O.A. designed the study, recruited patients, performed flow cytometric experiments and data analyses, provided intellectual input, and wrote the manuscript; H.S. provided intellectual input, performed data analysis, and wrote the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Oliver Andres, Universitätsklinikum Würzburg, Kinderklinik und Poliklinik, Josef-Schneider-St 2 / D31, 97080 Würzburg, Germany; e-mail: andres_o@ukw.de; and Harald Schulze, Universitätsklinikum Würzburg, Institute of Experimental Biomedicine, Chair I, Josef-Schneider-St 2 / D15, 97080 Würzburg, Germany; e-mail: harald.schulze@uni-wuerzburg.de.
References
Author notes
∗O.A. and H.S. contributed equally to this study.
Data are available on request from the corresponding authors, Oliver Andres (andres_o@ukw.de) and Harald Schulze (harald.schulze@uni-wuerzburg.de).
The full-text version of this article contains a data supplement.


![Reduced CD151 expression in neonates. Characteristics of preterm neonates <32 weeks GA, 32 to 36 weeks GA, >36 weeks GA (t), infants 28d-2a, children 2 to 13a, and adults >13a are displayed. (A) Representative images and (B) quantification of the different spreading phases of fixed term neonate and control platelets 45 minutes after differential interference contrast imaging (Zeiss Axiovert 200 inverted microscope [100×/1.4 oil objective]). (C-D) CD9 and (E-F) CD151 expression were assessed in whole blood using flow cytometry. (D) CD9 expression vs forward scatter (FSC) (F) PAC-1 binding after ADP (5 μM) stimulation vs CD151 expression. All graphs show the median ± 95% confidence interval. Differences were analyzed using Kruskal-Wallis-test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ns, nonsignificant.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/7/16/10.1182_bloodadvances.2023009824/2/m_blooda_adv-2023-009824-gr5.jpeg?Expires=1768133280&Signature=Jo9iyXHatuoUoPTNQqip~3J0L3MizZIU4j69GYd~BZEIweyRE52fFSdosL8JJ419WCudZYRBil-yD4Nk-k5ruNDjOmA87m9pIDCjp7xp01e83NBHQVKVH6juAlhLuOdUPXaEndgvHKDf9BmhdM6I5WFyMXTE7vbb9ohfW0hE7Mr0Mx3~pdxCu-RmsGA-zRioPihbgg2BqN96P1YINCImkwItt8PhNieVZMAhKw9U91QtQMilTswR1h2Wf9lVHP9DyCCsUDdU83Oq-ehDcrD7DN7rZEI5q18tJyaiTvZVgp3-89bguXgNJqoutiOXEKM5MJVevxxyzudsFz7GoYERUg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Reduced CD151 expression in neonates. Characteristics of preterm neonates <32 weeks GA, 32 to 36 weeks GA, >36 weeks GA (t), infants 28d-2a, children 2 to 13a, and adults >13a are displayed. (A) Representative images and (B) quantification of the different spreading phases of fixed term neonate and control platelets 45 minutes after differential interference contrast imaging (Zeiss Axiovert 200 inverted microscope [100×/1.4 oil objective]). (C-D) CD9 and (E-F) CD151 expression were assessed in whole blood using flow cytometry. (D) CD9 expression vs forward scatter (FSC) (F) PAC-1 binding after ADP (5 μM) stimulation vs CD151 expression. All graphs show the median ± 95% confidence interval. Differences were analyzed using Kruskal-Wallis-test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ns, nonsignificant.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/7/16/10.1182_bloodadvances.2023009824/2/m_blooda_adv-2023-009824-gr5.jpeg?Expires=1768289800&Signature=RECBrjo1kNiXSgqTR4yBiv-iwfjRDTgjEAOob8klhUYbjGaoy42BiNYhHML97pydUZgzJMPWo3FC4KTrExDyUBv-bohOvYIVDCp3M8R5njH1kNFPp-tZzxAiXTESAzzWyqJBc0ygkAbKBfVxBLPllf1YubmO7DNL~c6~yjqImfAZd9B7L9Q0sFMWJSEkR1DmKbGDn3SjHIVzFg~~yAegB~WZDX4FIdrAHqr5AqKVPnw3jOIepisgRXXlccEms4S~E7-GksDzjE9LXRZTxfExNlfHKIPrDZCWmsedtA8YLK1AFM1YmNVNg7a5S8UmHgVk4T-oz4GOY0H6h4MT5Oig6A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)