Key Points
Pediatric patients frequently experience chest pain and dyspnea after a PE, prompting frequent return ED visits.
Recurrent PE is uncommon and observed only in those with persistent or new thrombotic risk factors.
Abstract
Rates of pulmonary embolism (PE) in children have steadily increased over the past 2 decades. Patient outcomes after hospital discharge are poorly understood, and many patients experience recurrent or persistent chest pain or dyspnea, prompting a return to care. This retrospective cohort study of patients diagnosed with PE at a large children’s hospital over a 9.5-year period was performed to evaluate rates of return to the emergency department (ED) for PE-related symptoms, and to determine the utility of repeat computed tomography angiography (CTA) in this population. Ninety-six patients were diagnosed with PE during the study period. Forty-two percent of patients (n = 40) returned to the ED for PE-related symptoms and a total of 74 repeat CTAs were performed. Among those who had return visits, the mean number of return visits was 3 and the mean number of repeat CTAs was 1.8. The median time to return to the ED was 34 days. Logistic regression analysis identified increased age and female sex as risk factors for return ED visits. Eight percent of the cohort experienced PE recurrence. Recurrent PE was observed only in those with persistent or new thrombotic risk factors and was uncommon in those who remained on appropriate anticoagulation. Future work should focus on the development of a risk stratification system to identify patients at low risk of recurrence in order to minimize repeat CTA imaging.
Introduction
Pulmonary embolism (PE), although rare in children, is a serious thrombotic event associated with significant risk of mortality and morbidity. Although historical rates of pediatric PE approximate to 1 per 100 000 pediatric hospital discharges, rates of pediatric venous thromboembolism (VTE) have steadily increased over the past 2 decades, with rates of PE increasing out of proportion compared with the increase in rates of VTE in general.1-3 A study that analyzed data from the Pediatric Hospital Information Systems database identified a mean increase in pediatric PE incidence of 200% from 2001 to 2014.3 The etiology of this increasing incidence of PE is likely multifactorial and is hypothesized to be due to a combination of rising obesity rates, increased use of oral contraceptives for adolescents, and improved diagnostic testing via computed tomography angiography (CTA).4
As is the case with many uncommon pediatric conditions, the diagnostic and treatment strategies for pediatric PE are based on small pediatric studies or extrapolated from adult data. There are currently no validated clinical decision rules to aid providers in determining which patients should undergo diagnostic imaging, nor are there established risk stratification systems to guide disease management after diagnosis.5 As such, there is significant variability in diagnostic, treatment, and follow-up strategies among physicians.6
A particular area that requires further investigation is the natural history of children and adolescents with PE after hospital discharge. PE-related morbidity is difficult to quantify and has not been well described in pediatrics. Although, anecdotally, many patients experience chest pain and dyspnea for weeks, or months, after diagnosis, short- and intermediate-term outcomes after a PE are not well described in the literature. At our institution, we have noted that many patients present to emergency care again for persistent or recurrent symptoms, particularly in the first months after a PE. Because of the lack of a validated scoring system or discriminatory laboratory screening, children and adolescents may undergo frequent repeat imaging to rule out a recurrent PE. Long-term outcome data in pediatrics are also limited; recurrence rates ranging from 5% to 18% have been described in small case series.7-10 Although there are reports of chronic thromboembolic pulmonary hypertension and chronic dyspnea in the pediatric population after PE, incidence rates have not been reported.11 The lack of pediatric-specific literature regarding patient outcomes hinders physicians’ abilities to provide patients and families with appropriate anticipatory guidance and understand a patient’s true risk of PE-related morbidity.
With this single-center, retrospective cohort study, we sought to better understand the short- and intermediate-term outcomes of pediatric patients with PE, with the goal of identifying risk factors for their return to the emergency department (ED). We also sought to describe the use of CTA in this population and identify patients at highest risk for PE recurrence.
Methods
This study was deemed exempt by the institutional review board at the University of Pittsburgh, PA, and was conducted in accordance with the Declaration of Helsinki.
Pediatric patients (aged 0-21 years) with PE diagnosed between 1 January 2012 to 30 June 2021 were identified from UPMC Children’s Hospital of Pittsburgh pediatric coagulation service records. Patients with in situ pulmonary artery thrombosis were not included in this study. The pediatric coagulation service, or the primary-care team, is consulted with for all patients with PE by hospital standard. The electronic medical records of all patients with a diagnosis of PE over the study period were reviewed to search for return ED visits. This search included the Children’s Hospital of Pittsburgh ED as well as all University of Pittsburgh Medical Center–affiliated hospitals, including adult and community-based hospitals. Return ED visits were included in this analysis if the patient’s chief complaint included chest pain or tightness, shortness of breath, or respiratory distress. ED visits for unrelated complaints, such as orthopedic injuries or a patient’s chronic illness, were not included in the analysis. Using a standard data collection form, clinical and demographic data were collected from the electronic health record at the time of initial hospitalization for PE and subsequent related ED visits. The primary outcomes included the incidence of return ED visits and risk factors for return to the ED. The secondary outcomes that were evaluated included the frequency of CTAs performed and risk factors for PE recurrence.
Definitions
Diagnosis of PEs
Both initial PE and PE recurrences were determined via the review of radiology reports confirming new filling defects in pulmonary vasculature as well as electronic medical record documentation of the treating hematologist’s clinical determination. Filling defects that were recorded as chronic or stable by the radiologist were not considered to be PE recurrence.
Risk level of initial PE
Patients who presented with hypotension, cardiovascular, or respiratory failure were classified as being at high risk. Those with evidence of right ventricular dysfunction as evidenced by elevated troponin, electrocardiographic changes or echocardiogram findings (in the absence of hypotension), or cardiovascular or respiratory failure were classified as being at intermediate risk. Patients who were hemodynamically stable without evidence of right ventricular dysfunction were classified as being at low risk.
Thrombophilia
Thrombophilias investigated in our population included both congenital thrombophilias (factor V Leiden mutation, prothrombin gene mutation, antithrombin deficiency, protein C deficiency, and protein S deficiency) and the acquired antiphospholipid antibody syndrome (APS). Laboratory testing for APS included anticardiolipin antibodies, β2-glycoprotein I antibodies, dilute Russell viper venom time, hexagonal lipid neutralization, tissue thromboplastin inhibition, and reptilase time. The International Society on Thrombosis and Haemostasis criteria for APS, requiring a thrombotic event and persistence of at least 1 laboratory criterion over a period of 12 weeks, was used to diagnose APS. Diagnoses of protein C, protein S, or antithrombin deficiencies were made only in the presence of at least 2 abnormal levels. The decision to evaluate for thrombophilia was made by the treating hematologist. The Children’s Hospital of Pittsburgh uses a thrombophilia panel order set; however, treating hematologists may decide to omit, or add, tests based on clinical judgment.
Vital signs
Age-based vital-sign ranges per the American Heart Association Pediatric Advanced Life Support guidelines were used to assess vital signs. Vital sign assessment included heart rate, respiratory rate, pulse oximetry, and blood pressure measurements.
D-dimer
The Children’s Hospital of Pittsburgh reference range (normal, <0.4 μg/mL fibrinogen-equivalent units) was used to classify D-dimer as either normal or elevated.
Statistical analysis
Study variables were summarized using frequencies and percentages for categorical variables, and means and medians for continuous variables. Univariate analysis was conducted to identify risk factors for symptom persistence resulting in return to the ED. Variables analyzed were age, sex, history of thrombosis, body mass index (BMI), initial PE risk level, initial D-dimer value, presence of thrombophilia, initial anticoagulation medication, discharge anticoagulation medication, and duration of anticoagulation therapy. Covariates with a P value of <0.1 were included in a logistic regression model. Descriptive statistics were also used to explore risk factors for PE recurrence.
Results
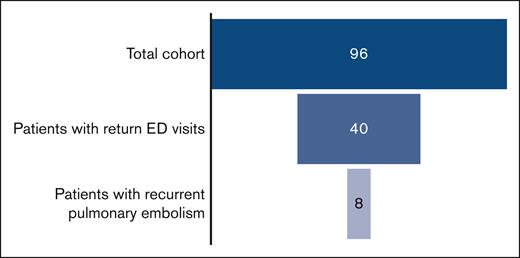
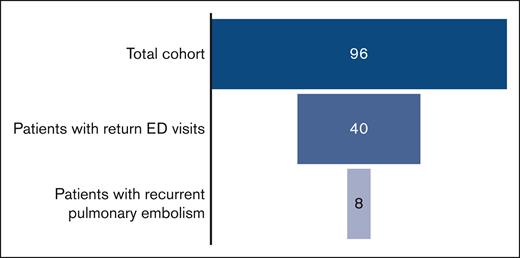
Over the study period of 9.5 years, 96 patients were treated at the UPMC Children’s Hospital of Pittsburgh for PE and were included in this study. Patient demographics and thrombotic risk factors are summarized in Table 1. The majority of patients in this cohort was female (59%) with a median age of 16 years at the time of initial PE. An identifiable thrombotic risk factor was identified in all but 2 patients. Regarding initial anticoagulation, 54% (n = 52) of patients received unfractionated heparin, and 42% (n = 40) received enoxaparin. Two patients began treatment with direct oral anticoagulants (DOACs), and 2 were unable to receive anticoagulation because of bleeding. Systemic thrombolysis was used in 2 patients. Most patients were discharged using enoxaparin (65%; n = 63). The remainder were discharged using DOACs (29%; n = 28) or warfarin (3%; n = 3). Sixty-eight percent (n = 65) received 3 or 6 months of anticoagulation, whereas 3 patients received <3 months of therapy because of bleeding symptoms. Eighteen percent (n = 17) were administered with long-term anticoagulation. Anticoagulation duration data were missing in 12% (n = 11) of patients.
Of the 96 patients in this cohort, 42% (n = 40) returned to the ED at least once for chest pain or respiratory chief complaints. Within this cohort, there were a total of 120 return ED visits for PE-related symptoms. Of those patients with a return visit, 60% (n = 24) returned more than once for similar complaints. Chest pain and dyspnea were present in 87% (n = 104) and 48% (n = 58) of the return visits, respectively. Of those whose symptoms prompted a return visit, 48% returned initially in the first 30 days after initial PE diagnosis, and 80% returned initially within the first 90 days. The mean number of return visits in this subgroup of returning patients was 3, with a range from 1 to 12 visits. A total of 74 repeat CTAs was completed in patients during return ED visits, with 24 patients having >1 repeat CTA performed. The mean number of repeat CTAs obtained was 1.8, with a range from 0 to 7 CTAs performed. Of the repeat CTAs in this cohort, 40% were completed within the first 90 days from initial PE diagnosis.
Female sex and increased age were identified, using univariate analysis, as risk factors for return to the ED (see Table 2). Based on obesity, thrombosis history, risk level of initial PE, initial and discharge anticoagulation type, initial D-dimer value, duration of anticoagulation, and presence of thrombophilia, no significant differences were identified in those with and those without a return ED visit. A subsequent multivariable logistic regression model that included age and sex demonstrated that the odds of having a return visit to the ED significantly increased as age increased (OR = 1.23; 95% confidence interval, 1.04-1.46; P = .014). The odds of having a return visit to the ED was also found to be significantly higher for females compared with that for males (OR = 2.82; 95% confidence interval, 1.14-6.98; P = .025).
In this study’s cohort, 8 PE recurrences were identified among the 40 patients who returned to the ED for chest pain or respiratory symptoms (Table 3). The median time to PE recurrence from the date of initial PE diagnosis was 17.5 months, with a range from 3 weeks to 51 months. All 8 PE recurrences occurred in patients with at least 1 persistent or new thrombotic risk factor. Risk factors included central venous cathethers, May Thurner syndrome, critical illness, obesity, systemic lupus erythematosus, APS, inherited thrombophilia, and immobility from surgery. Half of the recurrences occurred in patients who were on anticoagulation, with 2 patients having been on rivaroxaban and 2 on enoxaparin. Of the 2 patients receiving enoxaparin, 1 (patient 1) had an undetectable anti-Xa level at the time of PE recurrence. One recurrence occurred in a patient (patient 7) whose prophylactic enoxaparin was discontinued because of gastrointestinal bleeding. An additional 3 patients with PE recurrence were not receiving prophylactic or treatment anticoagulation.
Abnormal vital signs were more common in return visits with an identified PE recurrence than in return visits without a recurrence; at least 1 abnormal vital sign was present in 88% of the return visits with a PE recurrence compared with in 42% of return visits without a recurrence. D-dimer values were obtained in 28% (n = 34) of the 120 return ED visits. Comparison of D-dimer values between patients with PE recurrence and those without was not possible because only 1 patient with recurrence had a D-dimer value obtained at the time of recurrence diagnosis. Of those without PE recurrence who had D-dimer levels recorded during return visits, 15% had elevated values, with a range from 0.48 to 7.65 μg/mL fibrinogen-equivalent units.
Discussion
As rates of pediatric PE increase, gaining an improved understanding of the natural history of these thrombotic events becomes increasingly important. At our institution, we found a high rate of return ED visits for chest pain and dyspnea after an initial diagnosis of PE, indicating that many patients continue to experience symptoms despite appropriate treatment. This cohort’s rate of recurrent PE was 8%, similar to prior estimates in children.11 It is important to note that all patients who experienced a recurrent PE had new or persistent thrombotic risk factors.
This study demonstrates that more than a third of pediatric patients with PE will present again to the ED for chest pain or dyspnea after their initial diagnosis. This high number of return visits demonstrates the importance of providing more thorough anticipatory guidance to patients and families regarding symptom persistence. Many patients and families may expect the acute symptoms of a PE to resolve with the initiation of anticoagulation. However, symptoms more typically resolve slowly over a longer period, and some patients develop long-term exercise intolerance. Rates of chronic symptoms have not been fully explored in pediatrics; however, adult studies have found that more than half of patients with PE report dyspnea and functional limitations after a period from 6 months to 3 years.12 The true rate of persistent symptoms in children is likely higher than what was captured in our study because we did not identify those with persistent symptoms who presented for outpatient care rather than to the ED.
Patients presenting to the ED again for chest pain and dyspnea pose a diagnostic dilemma to clinicians. Although some degree of chest pain and dyspnea is expected while the pulmonary tissue heals, it is difficult to distinguish the expected symptoms associated with recovery from those associated with a new or worsening PE based on the history and physical exam alone.
In this cohort, a quarter of all patients with return visits had multiple repeat CTAs performed to evaluate for PE recurrence. The use of CTA is not without risk; statistical models have projected that 1 case of solid malignancy results from every ∼330 to 480 chest CTs performed in children.13 Large database studies have raised concern regarding the overuse of CTA, with 1 study identifying a CTA rate of 2.3% in all visits (adult and pediatric) among 16 EDs over a 3-year period, with a low positive yield rate of 3%.14,15 This work supports the use of scoring systems before obtaining diagnostic imaging. Unfortunately, existing screening tools such as the Wells criteria and D-dimer values have demonstrated limited utility among children, leaving clinicians with few options except direct imaging to evaluate for recurrent thrombus.16 Although a useful tool for ruling out PE in adults, D-dimer values have not demonstrated strong discriminatory power in pediatrics. Retrospective analyses report varying sensitivities of elevated D-dimer values in the diagnosis of pediatric VTE, ranging from 60% to 90%.17 A cohort study found that children with CTA-confirmed PEs were as likely to have normal D-dimer values as those who were PE-negative upon CTA.16 The use of D-dimer has not been specifically studied in the setting of concern for recurrent PE. Further prospective, multicenter evaluation of pediatric patients presenting with concern for initial or recurrent PE is required to elucidate whether a normal D-dimer value can be safely used to exclude a diagnosis of PE in children or adolescents.
Although the small number of recurrences in this cohort limits our ability to statistically determine risk factors for PE recurrence, the results of this study can be used to identify trends to guide further research. All 8 patients who experienced recurrence had at least 1 new or persistent thrombotic risk factor, with 6 of 8 patients having multiple risk factors. No patient whose initial PE was provoked by a modifiable and transient risk factor, such as oral contraceptives, experienced PE recurrence. Notably, half of the recurrences occurred in patients who were receiving anticoagulation therapy; 1 patient who was prescribed with enoxaparin had an undetectable anti-Xa level, suggesting medication nonadherence. One patient with APS and a BMI of 55 kg/m2 was receiving rivaroxaban for secondary thrombosis prevention; data suggest that the use of DOACs in patients with APS is associated with higher rates of VTE when compared with the use of warfarin and is not recommended.18-20 In addition, although recent guidance from the International Society on Thrombosis and Haemostasis suggests that rivaroxaban is a reasonable option for patients with BMIs of >40 kg/m2, there are limited data on patients with BMIs of >50 kg/m2.21 In this particular patient who was at high risk, an alternative anticoagulation strategy would have been beneficial. None of the recurrences occurred in patients on prophylactic anticoagulation.
Of the 8 PE recurrences, 6 occurred after the initial 90-day period from PE diagnosis. This is important to note, because many patients return to the ED soon after initial diagnosis when the pain from PE and anxiety regarding a recurrence is greatest. CTAs performed in the first 90 days after initial diagnosis accounted for 40% of all repeat CTAs, with only 2 of these CTAs (6.8%), revealing new areas of thrombus. This suggests that recurrent PE is unlikely in the short term. Vital signs abnormal for age were present at the time of PE recurrence in all but 1 patient and were less common in patients without a recurrence, suggesting that vital sign assessment continues to be helpful in assessing a patient’s risk of PE recurrence at the time of a return visit. Based on these findings, we suggest that in patients without new or persistent thrombotic risk factors, who present again to the ED with normal vital signs, deferral of CTA should be considered, particularly if the patient is presenting within the first 90 days of initial PE diagnosis. Decreasing CTA use in this population would decrease health care costs as well as radiation exposure and its associated downstream health effects in children and adolescents.
This study has several limitations. As a retrospective study using an institutional thrombosis list, the validity of these results is dependent on the inclusion of all cases of pediatric PE. Although it is the institutional standard in hematology to give consultation for or serve as the primary service for patients with PE, it is possible that some patients were not included in this database. Furthermore, we were only able to capture return ED visits when they occurred at the UPMC Children’s Hospital of Pittsburgh or other University of Pittsburgh Medical Center–affiliated hospitals. It is possible that there were more return visits than reported for patients presented again at smaller community EDs not accessible through our electronic medical record. This study is also limited by the small sample size and low number of PE recurrences, limiting our ability to make statistical conclusions regarding risk factors for recurrence. Multicenter work with a larger sample size would be required to statistically determine risk factors for recurrence. Finally, the role of the outpatient thrombosis nursing team was not assessed in this study. It is possible that patients who seek advice from their outpatient team regarding symptoms may receive either reassurance or encouragement to seek emergency care, which may have affected our results. Persistent or recurrent symptoms prompting return to emergency care is a common phenomenon after PE in children and adolescents. Clinicians should provide patients and families with appropriate anticipatory guidance regarding expected chest pain and dyspnea persistence during the healing phase, which may decrease ED visits. Future, prospective work focusing on identifying patients at low risk for PE recurrence through pediatric-specific risk stratification systems will improve emergency care clinicians’ judicious use of CTAs, thus limiting unnecessary radiation exposure in this population. Moreover, prospective observational studies to determine rates of chronic PE symptoms including exercise intolerance and dyspnea are required to gain a true understanding of PE-associated sequaela in children.
Acknowledgments
The authors thank Meghan McCormick and Char Witmer for their review of this manuscript and their thoughtful suggestions.
Statistical support was provided by The University of Pittsburgh Clinical and Translational Science Institute, supported by the National Institutes of Health through grants UL1 TR001857, KL2 TR001856, and TL1 TR001858. D.E.-S. is funded through National Institutes of Health T32 training grant 05.35129.5201.135886.00000; principle investigator, Enrico Novelli, University of Pittsburgh, Department of Internal Medicine.
Authorship
Contribution: D.E.-S. designed the research plan, performed data collection, analyzed the data, and wrote the manuscript; P.G. performed primary data analysis and provided statistical support; and J.D.C. supervised the research design, data collection, data analysis and interpretation, and manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dana Egan-Sherry, Division of Hematology/Oncology/Bone Marrow Transplant, UPMC Children’s Hospital of Pittsburgh, 4401 Penn Ave, Pittsburgh, PA 15224; e-mail: egansherrydl@upmc.edu.
References
Author notes
Data are available on request from the corresponding author, Dana Egan-Sherry (egansherrdyl@upmc.edu).