Key Points
Our study provides external validation of the PEGeD algorithm, which was developed to reduce CTPA in patients with suspected PE.
PEDeD safely ruled out PE, but caution is needed in patients with a negative PEGeD algorithm and D-dimer above the age-adjusted cutoff.
Abstract
Sequential diagnostic algorithms are used in the case of suspected pulmonary embolism (PE). The PEGeD study proposed a new diagnostic strategy to reduce the use of computed tomography pulmonary angiography (CTPA). We aimed to externally validate this diagnostic strategy in an independent cohort. We analyzed data from 3 prospective studies of outpatients with suspected PE. As per the PEGeD algorithm, patients were classified as having a low, moderate, or high clinical pretest probability (C-PTP). PE was excluded with a D-dimer <1000 ng/mL in case of low C-PTP and <500 ng/mL in case of moderate C-PTP. We assessed the yield and safety of this approach and compared them with those of previously validated algorithms. Among the 3308 evaluated patients, 1615 (49%) patients could have had PE excluded according to the PEGeD algorithm, without the need for imaging. Of these patients, 38 (2.3%; 95% confidence interval [CI], 1.7-3.2) were diagnosed with a symptomatic PE at initial testing or during the 3-month follow-up. On further analysis, 36 patients out of these 38 patients had a positive age-adjusted D-dimer. The risk of venous thromboembolic events among the 414 patients with a D-dimer <1000 ng/mL but above the age-adjusted D-dimer cut-off was 36 of 414 (8.7%; 95% CI, 6.4-11.8). We provide external validation of the PEGeD algorithm in an independent cohort. Compared with standard algorithms, the PEGeD decreased the number of CTPA examinations. However, caution is required in patients with a low C-PTP and a D-dimer <1000 ng/mL but above their age-adjusted D-dimer cut-off.
Introduction
Patients with suspected pulmonary embolism (PE) should be managed in accordance with validated diagnostic algorithms. These algorithms are based on the sequential use of the assessment of clinical probability, D-dimer measurement, and computed tomography pulmonary angiography (CTPA).1-4 Robust published data have consistently shown that the combination of a nonhigh pretest probability and a negative D-dimer measurement safely rule out PE without the need for radiologic imaging of the chest.5 Indeed, many prospective studies reported that the use of a nonhigh clinical probability, and a standard D-dimer cut-off set at 500 ng/mL allow to exclude PE in 30% of outpatients.6,7
To increase the diagnostic usefulness of this algorithm, the use of an age-adjusted D-dimer (AADD) cut-off in patients with a nonhigh clinical probability was prospectively validated and associated with a further 11.2% absolute reduction in the need for CTPA. Therefore, the use of an age-adjusted cut-off allows to safely rule out PE in ∼40% of patients, without thoracic imaging test.8
The PEGeD study used a modified Wells score along with D-dimer cutoffs adjusted to clinical probability rather than adjusted to age.9 This strategy was evaluated in a wide prospective Canadian study and was associated with a 17.6% absolute reduction in the use of CTPA as compared with a conventional strategy, without altering the safety outcome ie, the rate of venous thromboembolic events (VTE) at 3 months. However, before recommending the use of this strategy in everyday clinical practice, further external validation in an independent cohort is required. We aim to fill this knowledge gap by conducting a post hoc analysis of data from previous PE diagnostic studies.
Design and methods
Patients and setting
We used data from 3 prospective management outcome studies on PE diagnosis in outpatients with clinically suspected PE. All 3 studies aimed at validating diagnostic algorithms using various combinations of pretest probability assessment by the Geneva score, D-dimer measurement, compression ultrasound (CUS), and CTPA when needed,7,10,11 details of which have been published.7,10,11 The studies were conducted in France, Belgium, and Switzerland, and the inclusion period ranged from October 2000 to August 2006. All studies were approved by the ethics committees of participating centers. Written informed consent was obtained from all patients.
All patients included in these studies underwent a standardized prespecified diagnostic algorithm. Data on demographic, signs and symptoms, medical history, and VTE risk factors were collected on a standardized case report form. All the items necessary to compute the Geneva and the Wells score, including the likelihood of an alternative diagnosis to PE, were prospectively collected at the time of enrollment. The first step in all studies was pretest probability assessment by the Geneva score. Patients with a nonhigh ie, low or intermediate pretest probability had a high sensitivity D-dimer test, and PE was excluded in patients with D-dimer levels <500 ng/mL. Patients with either a high pretest probability or a nonhigh probability associated with a positive D-dimer underwent additional imaging comprising of CUS and CTPA or CTPA alone, based on specific study designs and objectives.7,10,11 Patients with PE confirmed through imaging were started with anticoagulant therapy. Patients in whom PE was ruled out were left untreated and followed up clinically for 3 months. They were instructed to report any new symptoms and objectively evaluated in case of suspected VTE. The primary outcome of the 3 studies was the rate of objectively confirmed adjudicated symptomatic VTE at 3 months.
The PEGeD algorithm
We conducted a post hoc analysis of the diagnostic performance of the PEGeD algorithm. The PEGeD algorithm consists of a clinical-probability adapted D-dimer threshold to exclude PE.12 The pretest probability assessment in the PEGeD study was performed using the Wells score. However, the cutoffs used to separate patients between the low and intermediate C-PTP categories were different from the original Wells scoring system. In the PEGeD study, patients with a Wells score of 0 to 4 points (instead of 0-1.5 points in the original Wells model) were categorized as low C-PTP, and a D-dimer cut-off of 1000 ng/mL was used to exclude PE in these patients.12 Patients with a Wells score of 4.5 to 6 points (instead of 2.0-6.0 points) were categorized as moderate C-PTP, and the standard D-dimer cut-off of 500 ng/mL was used to exclude PE. Patients with a Wells score ≥ 6.5 points were considered as having high C-PTP and were referred directly for CTPA.
Data analysis
Using prospectively collected items of the Wells score, patients were categorized into 3 C-PTP groups according to the PEGeD model described in “The PEGeD algorithm.” We determined the proportion of patients in each category. We first computed the overall failure of the strategy. Then, according to the PEGeD strategy, D-dimer cutoffs of 1000 ng/mL or 500 ng/mL were applied in patients with low C-PTP and moderate C-PTP, respectively. The proportion of patients with a negative D-dimer level in each category was computed. We assessed the failure rate ie, the VTE rate at 3 months in patients who would have been left untreated after PE exclusion by C-PTP and D-dimer results according to the PEGeD model. We further estimated the failure rate among patients in the low c-PTP group with D-dimer >500 ng/mL but <1000 ng/mL cut-off, and the failure rate in patients with D-dimer above the age-adjusted cut-off and <1000 ng/mL cut-off. Given the low rate of missing values, complete data analysis was used, excluding records with missing values for key variables. No imputation was performed.
Results
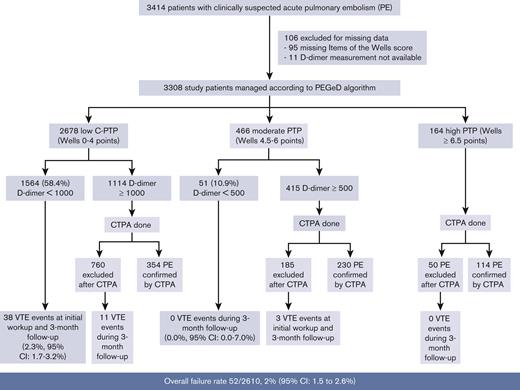
Altogether, 3414 patients were included in the 3 studies. Owing to missing values for some items of the Wells score (likelihood of an alternative diagnosis [n = 81], heart rate [n = 8], signs and symptoms of deep vein thrombosis (DVT) [n = 2], previous VTE [n = 2], and cancer [n = 3]), 95 patients were excluded from the analysis (2.8%). Moreover, D-dimer was not available in 17 patients (11 patients with moderate C-PTP and 6 with high C-PTP). The final population sample was therefore 3308 patients. PE was confirmed in 730 of 3308 patients (22.1%). General characteristics of patients are displayed in Table 1.
The PEGeD algorithm: diagnostic yield and safety
Applying the Wells score cutoffs used in the PEGeD study, 2678 (81.1%) patients would have been classified as low (Wells 0-4.0 points), 466 (14.1%) as moderate (Wells 4.5-6.0 points), and 164 (4.9%) as high C-PTP (Wells ≥ 6.5 points). PE prevalence was 392 of 2678 (14.6%) in patients with low C-PTP, 230 of 466 (49.4%) in patients with moderate C-PTP, and 114 of 164 (69.5%) in patients with high C-PTP. The overall failure rate of the strategy was 52 of 2610 (2%; 95% confidence interval [CI], 1.5-2.6).
1564 out of the 2678 patients with low C-PTP had a D-dimer <1000 ng/mL, and 51 out of the 466 patients with moderate C-PTP had a D-dimer <500 ng/mL. Altogether, 1615 out of 3308 (49%) patients had a negative D-dimer level and had PE excluded without imaging based on the PEGeD strategy (Figure 1). Of these, 38 were diagnosed with PE at baseline or during follow-up. All cases were diagnosed in patients with low C-PTP and D-dimer <1000 ng/mL. The most proximal PE location was troncular in 2, lobar in 4, segmental in 21, and multiple subsegmental in 5, but the 6 remaining patients were diagnosed based on a proximal DVT on lower limb CUS. The failure rate of the PEGeD algorithm in this group was 38 of 1615, 2.3% (95% CI, 1.7-3.2). Using the conventional 2-level Wells score and a fixed D-dimer cut-off of 500 ng/mL, 985 out of 3308 (29.8%; 95% CI, 29.8-31.4) patients would have been managed without imaging with no failure (0.0%; 95% CI, 0.0-0.4).
Comparison between a fixed 1000 ng/mL cut-off and the AADD cut-off in patients with low C-PTP
Of the 1564 patients with low C-PTP and D-dimer <1000 ng/mL, 985 had D-dimer <500 ng/mL and 579 had D-dimer ≥500 ng/mL. All 38 PE were diagnosed in patients with D-dimer ≥500 ng/mL and <1000 ng/mL, corresponding to a proportion of confirmed PE of 38 of 579 (6.6%; 95% CI, 4.8-8.9). Using the AADD cut-off, 1150 of 1564 patients had a negative AADD, of whom 2 (0.2%; 95% CI, 0.0-0.6) had PE. In the 414 patients with a D-dimer above their AADD cut-off but <1000 ng/mL; PE was confirmed in 36 of 414 (8.7%; 95% CI, 6.4-11.8) (Figure 2).
Discussion
Our study provides external validation of the PEGeD algorithm. The overall failure rate of the PEGeD algorithm in our cohort was 52 of 2610 (2%; 95% CI, 1.5-2.6). This is in line with the failure rate reported in a recent Individual Patient Data MetaAnalysis (IPDMA)(ie, 2.8%; 95% CI, 2.3-3.5).13 Using this diagnostic strategy, 49% of patients could have had PE excluded at presentation without the need for further imaging (vs 30% using a conventional strategy of a 2-level Wells score and a fixed 500 ng/mL D-dimer cut-off). This increased efficiency was however achieved at the expense of 38 missed diagnoses, all in patients with a low C-PTP and a D-dimer between 500 ng/mL and 1000 ng/mL. Indeed, in our analysis, all missed diagnoses occurred in patients in whom the new D-dimer cut-off of 1000 ng/mL was used, of whom all had a D-dimer >500 ng/mL. The failure rate among patients with low C-PTP (Wells score, 0-4 points) and a D-dimer <1000 ng/mL was 38 of 1564 (2.4%; 95% CI, 1.7-3.3). Almost all these patients (36 of 38) had a D-dimer between their age-adjusted cut-off and 1000 ng/mL. Therefore the failure rate of the PEGeD algorithm in patients with a low c-PTP and D-dimer level between their AADD cut-off and 1000 ng/mL was high (36 of 414 [8.7%]; 95% CI, 6.4-11.8). This means that among the 414 patients with D-dimer levels above the age-adjusted cut-off in whom imaging would be avoided by the PEGeD model, 1 in 11 would have a missed PE diagnosis. If confirmed, this failure rate would preclude the use of the PEGeD algorithm in settings similar to ours ie, European emergency rooms would assess patients with clinically suspected PE, with a typical prevalence of confirmed PE ∼15% to 20%.
On the other hand, the efficiency gain of the 1000 ng/mL cut-off in patients with low C-PTP compared with the AADD cut-off in the same subgroup of patients was a hypothetical 15.5% (1564 of 2678-1150 of 2678) decrease in the proportion of patients requiring CTPA. Altogether, as with other strategies assessing higher D-dimer cutoffs in patients with lower pretest clinical probability, the increase in efficiency is once again achieved at the expense of a decrease in safety.9,14
Notably, articles reporting on recent diagnostic algorithms usually report the global failure rate rather than failure rates in patients in whom the diagnostic management was modified by the new strategy. This includes the subgroup of patients with a low c-PTP and a D-dimer between 500 ng/mL and 1000 ng/mL in the PEGeD study or the subgroup of patients without YEARS items and a D-dimer level between 500 ng/mL and 1000 ng/mL in the YEARS study.15 Our study highlights the importance of providing specific subgroups’ failure rates for patients in whom the new diagnostic algorithm alters diagnostic management compared with previously validated algorithms. The global failure rate dilutes the actual impact of the strategy by including many patients among whom the new strategy is not used and safety has already been established (eg, patients with nonhigh PTP and D-dimer <500 ng/mL).
This issue has led to experts’ discussions on the optimal reporting of safety results of VTE diagnostic strategies.16,17 The debate remains open on whether the reported failure rate of a diagnostic strategy should focus on the whole cohort of patients with suspected PE or on the subgroup of patients undergoing the interventional part of the strategy. The latter has the advantage of reporting the actual results in the subgroup of patients of interest ie, those in whom the diagnostic management is altered using the new strategy compared with using the previously validated strategy.16,17 Admittedly, much larger studies need to be performed to reach narrow enough estimates in each subgroup. Moreover, it is difficult to determine the maximal acceptable failure rate in such subgroups.13,18 The 2% maximal failure rate proposed by the SSC to define a safe strategy was intended to be used for the overall failure rate of the strategy and may not apply when considering failure rate of restricted subgroups within a diagnostic study. For example, patients with high probability cancer may have a higher incidence of de novo VTE during follow-up.15
Over the last 10 years, investigators have tried to maximize the diagnostic efficiency of the D-dimer test, with the hope of avoiding as many CTPA as possible, in the context of an overall decreasing prevalence of confirmed disease among suspected patients.8,9,12,19-21 The quest for the optimal tradeoff between maximal efficiency and preserved safety is challenging. It appears that one of the main determinants of this balance is PE prevalence in the tested population. PE prevalence was 22% in our study vs 7.4% in the PEGeD cohort. This likely represents the central explanation for the discrepancy in safety observed between our study and the original PEGeD study. We made similar observations when externally validating the YEARS algorithm in our cohort.14
In a recent systematic review of PE diagnostic studies, Germini et al showed a wide variation in PE testing and PE prevalence across European and North American countries. This work highlighted the much higher prevalence of confirmed PE among suspected patients in Europe compared to those in North America.22 The authors suggest that a consequence of these variations is that some algorithms might prove safe in North America but not in Europe. Unfortunately, this systematic review was not able to provide figures for other continents, and, therefore, clinicians should likely determine their comfort level with the use of newer algorithms based on their global PE prevalence or, if unknown, on their clinical setting (ie, primary care, emergency room, or inpatients).13,18
Limitations of our study include missing values, of which the majority was documentation of the item, alternative diagnosis and PE as the most likely diagnosis of the Wells score. Missing data, however, was concerned with only a low proportion of the whole cohort. Moreover, although it was prospectively collected, the likelihood of an alternative diagnosis to PE had no role in the diagnostic management, which could have affected the assessment, of this variable, by the attending physicians. Another limitation is the retrospective nature of our analysis. We could not have predicted the outcomes of the 38 patients with negative “missed” PEs, had they not undergone CTPA at baseline in accordance with the PEGeD algorithm. Notably, none of these were isolated subsegmental PEs.
Another limitation is that the data of patient population used in this analysis were collected before 2006, and PE prevalence in our cohort is likely higher than the current prevalence even in Europe. For example, 13% in the Netherlands in 2018.9
In conclusion, our study provides external validation data for the PEGeD algorithm. Overall, the PEGeD algorithm appears to safely exclude PE, but its broad implementation in clinical practice deserves more caution among populations with a high PE prevalence than what was observed in the original PEGeD study.
Acknowledgments
The CT-EP2 and the CT-EP4 studies were supported by the following grants from the Swiss National Research Foundation: 32-61773.00 and 32-00BO-105988. The CT-EP3 study was supported by a grant from the Hertz Foundation.
G.L.G. holds the chair on diagnosis of venous thromboembolism in the Department of Medicine at the University of Ottawa and a clinician scientist award from the Heart and Stroke Foundation.
Authorship
Contribution: H.R.-E., M.R., and G.L.G. contributed to the design of the study and the acquisition and interpretation of the data; P.-M.R., F.V., and O.S. contributed to the interpretation of the data; H.R.-E. drafted the manuscript; H.R.-E., M.R., and G.L.G. had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis; and all authors revised the work critically for important intellectual content and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Helia Robert-Ebadi, Division of Angiology and Hemostasis and Faculty of Medicine, Geneva University Hospitals, 4, rue Gabrielle-Perret-Gentil, 1205 Geneva, Switzerland; e-mail: helia.robert-ebadi@hcuge.ch.
References
Author notes
Data are available on Yareta (https://yareta.unige.ch/home).
The full-text version of this article contains a data supplement.