TO THE EDITOR:
In the clinic setting, there is now a plethora of Bruton tyrosine kinase inhibitors (BTKis), with either covalent or noncovalent binding to BTK, for malignant and autoimmune indications. Which BTKi is optimal for specific indications currently remains unclear; both relative efficacy and toxicities of an individual BTKi will depend on its precise mechanism of action. Chemical variables include the mechanism of binding of the BTKi within the adenosine triphosphate binding site of BTK and, in the case of covalent inhibitors, the mechanisms and kinetics of binding of the BTKi to the exposed cysteine-481 residue.
In the context of prolonged BTKi treatment required for chronic lymphocytic leukemia (CLL), variations in the binding of BTKi to BTK may be reflected in differences in patterns of BTK mutation. We read, with interest, the 2 recent publications in Blood Advances by Blombery et al1 and Naeem et al,2 and the recent publication in the New England Journal of Medicine by Wang et al,3 describing patterns of BTK mutations associated with the covalent BTKi zanubrutinib and the noncovalent BTKi pirtobrutinib.
Patients with CLL treated with the first-in-class BTKi ibrutinib4-9 or second-generation BTKi acalabrutinib10 predominantly relapse with BTK C481S mutations, resulting in reduced binding affinity of the covalent BTKi to BTK. In comparison, zanubrutinib1,11 and pirtobrutinib3 demonstrated different BTK mutational patterns (supplemental Table 1). Blombery et al showed that patients with progressing disease while receiving zanubrutinib had enrichment for BTK L528W mutations.1,12 Unlike C481S, which results in a kinase that retains activity, L528W prevents both the binding of covalent inhibitors and the binding of adenosine triphosphate, resulting in a “kinase-dead” protein. L528W and other non-C481 mutations within the kinase domain (including V416L, A428D, M437R, and T474I) have also been described at progression with pirtobrutinib.3 In the case of 2 inactivating C481 mutations (C481F and C481Y), signaling to PLCG2 may be maintained via a kinase-independent activation of hematopoietic cell kinase,13 although it is unclear whether this bypass mechanism is initiated with L528W mutation.
In 9 patients with progressing disease while receiving pirtobrutinib in the BRUIN trial, multiple non-C481 BTK mutations were identified in 78% of the patients.14 In contrast, non-C481 mutations were only rarely observed in patients with CLL treated with ibrutinib and acalabrutinib. Only 5% (6 out of a total of 115 previously reported patients with CLL) had disease progression with non-C481 mutations while receiving ibrutinib4-9 (supplemental Table 2). In 67% of these patients, non-C481 BTK mutations occurred with concurrent C481 or PLCG2 mutations, and all but 1 of these detected non-C481 mutations were at variant allele frequencies <8%.
Here, we report a series of 9 patients with CLL whose condition progressed while receiving the covalent BTKi tirabrutinib15 and who show a further distinct BTK mutational profile. BTK mutations were identified via both whole-exome sequencing and digital droplet polymerase chain reaction (ddPCR) and are summarized in Figure 1A and Table 1. All 9 patients with CLL who relapsed while receiving tirabrutinib had disease progression rather than Richter transformation; the duration of tirabrutinib administration before disease progression is shown in supplemental Figure 1, and clinical information is outlined in supplemental Table 3. There was a significant enrichment of non-C481 BTK mutations in patients treated with tirabrutinib compared with those treated with ibrutinib but not with those treated with zanubrutinib (supplemental Table 1). No PLCG2 mutations were detected.
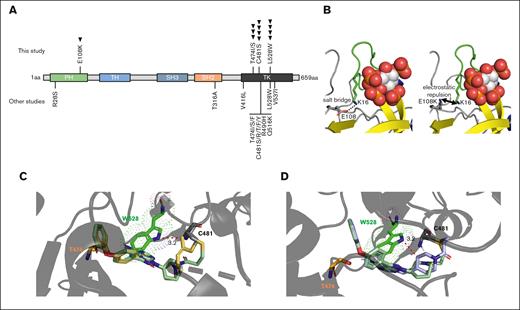
BTK mutations in patients with CLL receiving tirabrutinib. (A) Schematic domain structure of BTK with mutations identified in this study (top) (with each arrow denoting a single patient) and mutations identified in other studies of patients treated with ibrutinib, acalabrutinib, zanubrutinib, and pirtobrutinib (bottom). (B) Molecular modeling of the BTK pleckstrin homology (PH)-domain E108K mutation. Ins(1,3,4,5)P4 are shown as spheres, PH-domain residues of interest are shown as sticks. The β1-β2 loop, which forms part of the Ins(1,3,4,5)P4 binding site is indicated in green. (Left) E108 and K16 form a salt bridge, which stabilizes the Ins(1,3,4,5)P4 binding site. (Right) the E108K mutation interrupts the salt bridge to K16 and is likely to destabilize the Ins(1,3,4,5)P4 binding site because of electrostatic repulsion via K16 of the β1-β2 loop. (C-D) Molecular modeling of ibrutinib (pale green), tirabrutinib (yellow), and zanubrutinib (purple) bound to BTK. BTK W528 disrupts the binding of BTKi to BTK, with additional steric clash observed with tirabrutinib and zanubrutinib (denoted by red dotted lines). Mutated W528 is shown in bright green, and T474 shown in orange.
BTK mutations in patients with CLL receiving tirabrutinib. (A) Schematic domain structure of BTK with mutations identified in this study (top) (with each arrow denoting a single patient) and mutations identified in other studies of patients treated with ibrutinib, acalabrutinib, zanubrutinib, and pirtobrutinib (bottom). (B) Molecular modeling of the BTK pleckstrin homology (PH)-domain E108K mutation. Ins(1,3,4,5)P4 are shown as spheres, PH-domain residues of interest are shown as sticks. The β1-β2 loop, which forms part of the Ins(1,3,4,5)P4 binding site is indicated in green. (Left) E108 and K16 form a salt bridge, which stabilizes the Ins(1,3,4,5)P4 binding site. (Right) the E108K mutation interrupts the salt bridge to K16 and is likely to destabilize the Ins(1,3,4,5)P4 binding site because of electrostatic repulsion via K16 of the β1-β2 loop. (C-D) Molecular modeling of ibrutinib (pale green), tirabrutinib (yellow), and zanubrutinib (purple) bound to BTK. BTK W528 disrupts the binding of BTKi to BTK, with additional steric clash observed with tirabrutinib and zanubrutinib (denoted by red dotted lines). Mutated W528 is shown in bright green, and T474 shown in orange.
In total, 4 patients had a C481 mutation detected at relapse, of whom 1 had an additional T474I mutation, and 2 had both a T474I and L528W mutation. Only 1 patient exhibited a BTK C481 mutation as an isolated event. No patient had a T474 mutation in the absence of a C481 mutation. T474 mutations result in increased BTK kinase activity and decreased sensitivity to both covalent and noncovalent BTKi.16 In 1 patient, T474I was acquired with an E108K mutation within the pleckstrin homology domain. Modeling of E108K suggests an interruption of the salt bridge to BTK K16, with likely destabilization of the Ins(1,3,4,5)P4 binding site due to electrostatic repulsion via K16 of the β1-β2 loop (Figure 1B). The previously described R28S has structurally similar consequences,6 and such pleckstrin homology domain mutations will prevent the accumulation of activated BTK at the inner leaflet of the cell surface membrane, significantly impairing kinase activity.
In 2 male patients, L528W was observed without accompanying C481 and T474 mutations, with cancer cell fractions of 86.8% and 91%, suggesting a dominant resistance mechanism. Cancer cell fractions were calculated as variant allele frequencies via whole-exome sequencing, taking into account the copy number state of the mutation site and, thus, were unaffected by copy number variations of the X chromosome.
C481S and T474I mutations were detected in a sample from a patient with CLL (patient identified as CLL-6) 36 months before clinical relapse (supplemental Figure 1). Three patients treated with tirabrutinib lacked both BTK and PLCG2 mutations, highlighting the role of alternative/nongenetic mechanisms of resistance. PLCG2 mutational status of zanubrutinib-treated CLL has not been described but have been observed with pirtobrutinib (22% of the patients who relapsed).3
Besides mutations to BTK, no other consistent genetic aberrations (including single-nucleotide variants or copy number aberrations) were detected that are considered to be important in the development of resistant disease (supplemental Figure 2), even for the 3 patients without detectable BTK and PLCG2 mutations. Differential gene expression profiling was also carried out, with no consistent changes occurring at relapse in these cases (data not shown).
Because of the similarities between tirabrutinib and zanubrutinib with respect to the higher percentage of patients acquiring L528W mutations compared with ibrutinib, we compared the effect of BTK L528W on the binding of tirabrutinib, zanubrutinib, and ibrutinib to BTK in the context of mutated L528W using molecular modeling. We, and others, have shown that L528W caused steric hindrance, by which covalent BTKis are pushed away from the C481 covalent binding site, disrupting covalent interactions of all 3 inhibitors16 (supplemental Figures 3 and 4). However, additional steric hindrance was caused by W528 when tirabrutinib and zanubrutinib, compared with ibrutinib, were bound to BTK. This is because of a predicted additional steric clash of W528 with the methyl group present in tirabrutinib (Figure 1C) and piperidine present in zanubrutinib (Figure 1D), both of which were 0.6Å closer to W528 compared with the groups present in ibrutinib.
Although individually based on small numbers of patients, these data collectively suggest that different BTKis may be associated with different mutational patterns of both BTK and PLCG2, which may be reflected in differential modes of the binding of the BTKi to BTK. We have demonstrated that tirabrutinib appears to induce C481, T474, and L528 mutations at approximately equal frequencies (44%, 33%, and 44% respectively). Of the patients who developed a C481 mutation, 75% additionally developed a T474 mutation or both T474 and L528 mutations. This is different from patients treated with ibrutinib, in whom C481 mutations were predominant genetic drivers of resistance, and alternative BTK mutations were rare.
These data may have clinical implications because patients with disease progressing while receiving covalent BTKi with non-C481 mutations may have suboptimal responses with subsequent pirtobrutinib treatment, as demonstrated by Blombery et al,1 presenting issues of cross-resistance. This highlights the need for the clinical development of high-sensitivity assays for the detection of non-C481 BTK mutations.
Acknowledgments: The authors thank their patients and the research nurses at the Hope Clinical Trial Unit, Leicester Royal Infirmary. The authors used the ALICE High Performance Computing Facility at the University of Leicester for this research.
This work was supported by funds from the Scott Waudby Trust, the Hope Against Cancer charity, Cancer Research UK in conjunction with the UK Department of Health on an Experimental Cancer Medicine Centre grant (C10604/A25151), Leicester Drug Discovery and Diagnostics under the University of Leicester Institute for Precision Health (MRC - Impact Acceleration Account), the University of Leicester KEIPOC, National Institute for Health and Care Research Leicester Biomedical Research Centre, and Gilead Sciences.
Contribution: R.A.J. performed research, analyzed data, performed the bioinformatics analysis, and wrote the manuscript; C.S.T. analyzed data and performed bioinformatics analysis; S.J. performed research, analyzed data, and wrote the manuscript; C.M.C. and S.L. performed research; R.G.B. and R.S. performed and analyzed molecular modeling–based analyses; V.M.S. analyzed data and wrote the manuscript; C.F. provided clinical samples and treated patients; H.S.W. and M.J.S.D. supervised the study, treated patients, and wrote the manuscript; and all authors approved the final version.
Conflict-of-interest disclosure: S.J., H.S.W., and M.J.S.D. have received research funding from Gilead Sciences. H.S.W. and M.J.S.D. have received research funding from BeiGene and Loxo Oncology. The remaining authors declare no competing financial interests.
Correspondence: Martin J. S. Dyer, Department of Genetics and Genome Biology, The Ernest and Helen Scott Haematological Research Institute, University of Leicester, Henry Wellcome Bldg Room 3/57, Lancaster Rd, Leicester LE1 9HN, United Kingdom; e-mail: mjsd1@leicester.ac.uk.
References
Author notes
Data are available on request from the corresponding author, Martin J. S. Dyer (mjsd1@leicester.ac.uk).
The full-text version of this article contains a data supplement.