Key Points
TRES is a novel prognostic score and is associated with OS in patients with lymphoma.
TRES score is associated with lymphoma-specific survival and death from other cancers in older adults.
Abstract
For patients with non-Hodgkin lymphoma (NHL), formal comorbidity assessment is recommended but is rarely conducted in routine practice. A simple, validated measure of comorbidities that standardizes their assessment could improve adherence to guidelines. We previously constructed the 3-factor risk estimate scale (TRES) among patients with chronic lymphocytic leukemia (CLL). Here, we investigated TRES in multiple NHL subtypes. In the surveillance, epidemiology, and end results–Medicare database, patients with NHL diagnosed from 2008 to 2017 were included. Upper gastrointestinal, endocrine, and vascular comorbidities were identified using ICD-9/ICD-10 codes to assign TRES scores. Patient characteristic distributions were compared using χ2 or t test. Association of mortality and TRES score was assessed using Kaplan-Meier and multivariable Cox regression model for competing risk. A total of 40 486 patients were included in the study. Median age was 77 years (interquartile range [IQR], 71-83 years). The most frequent NHL subtypes were CLL (28.2%), diffuse large B-cell (27.6%), and follicular lymphoma (12.6%). Median follow-up was 33 months (IQR, 13-60 months). TRES was low, intermediate, and high in 40.8%, 37.0%, and 22.2% of patients, corresponding to median overall survival (OS) of 8.2, 5.3, and 2.9 years (P < .001), respectively. TRES was associated with OS in all NHL subtypes. In multivariable models, TRES was associated with inferior OS and NHL-specific survival. TRES is clinically translatable and associated with OS and lymphoma-specific survival in older adults with NHL.
Introduction
Non-Hodgkin lymphoma (NHL) is the most common hematologic malignancy among adults, with ∼72 000 new cases and 20 000 deaths recorded annually in the United States.1 The NHLs are a diverse group of related lymphoid malignancies with a highly variable course ranging from indolent to aggressive. The median age at diagnosis is ∼70 years, and most patients have comorbid medical conditions. Comorbidities are associated with survival in NHL, and various methods of measuring comorbidities have been described.2-8 The National Comprehensive Cancer Network recommends assessing comorbidities when selecting treatment for patients with B-cell or T-cell NHL.9,10 However, formal assessment of comorbidities in clinical practice is uncommon.
Of all NHL subtypes, chronic lymphocytic leukemia (CLL) is the one in which assessment of comorbidities is arguably most studied. CLL accounts for ∼20% of annual NHL cases in the Unites States. Median age at CLL diagnosis is 71 years, and overall, being similar to NHL, most patients have comorbidities at diagnosis.11,12 The international workshop on CLL recommends formal assessment of comorbidities in all patients with CLL enrolled in clinical trials; the National Comprehensive Cancer Network recommends assessing comorbidities in all patients with CLL when selecting treatment.13,14 Neither of the groups endorse a specific measure of comorbidity; however, in CLL, comorbidity is most frequently measured using the cumulative illness rating scale, which includes 14 comorbidity categories.15
We previously developed the CLL comorbidity index (CLL-CI), a 3-factor comorbidity scale that stratifies patients into low-, intermediate-, and high-risk categories.16 To develop the CLL-CI, we first identified the cumulative illness rating scale comorbidity categories most strongly associated with event-free survival, including vascular, endocrinal, and upper gastrointestinal associated comorbidities.16 We then combined these categories to create the CLL-CI, in which patients with no involved organ system have low risk, 1 involved organ system have intermediate risk, and 2 or 3 involved organ systems have high risk. We then validated the CLL-CI in patients with CLL using the Danish National Cancer Registry.17 In both our original study and the validation study, the CLL-CI score was associated with overall survival (OS) and event-free survival. The association of the CLL-CI score with the OS in other NHL subtypes has not been studied.
Here, we present an analysis of the results of CLL-CI in patients with NHL from the surveillance, epidemiology, and end results (SEER)-Medicare database. Because this study included patients with CLL and other NHL subtypes, we now call the CLL-CI “the 3-factor risk estimate scale” (TRES). CLL-CI and TRES scores are assigned identically. We hypothesized that the TRES score is associated with OS and lymphoma-specific survival in patients with NHL. Assessment of comorbidities among patients with NHL is not standardized, and many available methods are prohibitively complex.4,8,18 The TRES score could significantly improve the care of older adults with NHL and comorbidities by providing a standardized, easy-to-use scale that could aid in prognostication, treatment selection, and future clinical trial design.
Methods
Study population and data source
Data were obtained from the SEER-Medicare database, which is composed of a linkage between population-based cancer registry data from 21 SEER areas with Medicare administrative data. The SEER program covers ∼35% of the US population, and the Medicare program provides benefits to 94% of Americans aged ≥65 years.19,20
We used the SEER-Medicare cancer file to identify patients with NHL (ICD-O-3/World Health Organization recodes 25012, 33041, and 33042), diagnosed from 2008 to 2017. Patients were required to have a diagnosis of NHL confirmed pathologically, not by death certificate/autopsy. The cohort was then limited to patients aged ≥66 years at diagnosis. Given our intention to study comorbidity, we limited our cohort to individuals with continuous enrollment in Medicare parts A, B, and D from 12 months before to 12 months after cancer diagnosis or until death, if the patient died within 12 months. Patients enrolled in an Health Maintenance Organization (HMO) during the same time window were excluded.
Comorbidities, treatments, and covariates
Clinical and demographic characteristics were deidentified and obtained from the SEER-Medicare cancer file and included histologic subtype, year of diagnosis, other cancers diagnosed before or after NHL diagnosis, sex, age at NHL diagnosis, cancer stage, race and ethnicity, geographic region, marital status, median income of residents in census tract, and the percentage of residents in census tract with <12 years of education.
The TRES score was calculated on the basis of comorbidities coded in the 12 months before NHL diagnosis using the same medical conditions and scoring system that was used for the CLL-CI.16,17 Comorbidities were identified using ICD-9/ICD-10 diagnosis and procedure codes from inpatient, outpatient, and physician claims and prescription drug information from Medicare part D claims (Table 1). Patients were classified in the following groups: low risk (TRES score 0), intermediate risk (TRES score 1), and high risk (TRES score 2-3).
Treatment information registered from 2007 to 2019 was extracted from Medicare claim files. NHL treatments were identified using ICD-9/ICD-10 diagnosis and procedure codes; health care common procedure coding system codes from inpatient, outpatient, and physician claims; and prescription drug information from Medicare part A, B, and D claims (supplemental Table 1). On the basis of the first treatment received after the NHL diagnosis and before any later diagnosis of another cancer, we grouped initial treatment for NHL into 6 categories: none, chemotherapy, immunotherapy, chemo-immunotherapy, radiation therapy, and targeted therapy.
The treatment listed in the first medical claim for chemotherapy, immunotherapy, or targeted therapy after cancer diagnosis was defined as the first line of treatment. A treatment line was considered to have ended when a patient had not received any treatment for ≥90 days. Any treatment started after a treatment gap of ≥90 days was considered a subsequent treatment line. The regimen in each treatment line was defined as the drug combination received within the initial 7 days of treatment.
The outcome variables in our study were overall mortality and cause-specific mortality. Information on the cause of death was extracted from the SEER registry record. We evaluated mortality based on the cause, ie, NHL, non-NHL cancer, and noncancer causes. Survival was calculated from the date of NHL diagnosis to either death or until 31 December 2018. Time from cancer diagnosis to initiation of the first-line treatment and time between initiation of the first-line and the second-line treatments were calculated and analyzed as the time to event.
Statistical analyses
χ2 or t test was used to compare patients’ characteristic distributions between TRES-risk groups. Kaplan-Meier estimate and multivariable Cox regression model for competing risk were used to test the association between patients’ mortality and TRES risk. All statistical tests were 2-sided with statistical significance level at 0.05.
Given that there could be confounding factors, such as age, race and ethnicity, year of diagnosis, and other sociodemographic factors driving the cancer treatment and the survival, we also conducted a Cox proportional hazards regression with inverse probability weighting based on patients’ NHL type. The outcome variable for the propensity score is the TRES score. The score weights were based on propensity scores calculated using the following variables: year of diagnosis, age at diagnosis, race and ethnicity, marital status at diagnosis, urban or rural code at diagnosis, type of NHL, census tract percentage with some college education or higher, and census tract percentage of residents living below poverty line. The use of the inverse probability weighting approach has been shown to reduce the impact of the confounding factors on the OS.21,22 The hazard ratios (HRs), corresponding 95% confidence intervals (CIs), and P-values are provided.
The study was approved by the institutional review board at The University of Texas MD Anderson Cancer Center and was conducted under a data-use agreement with the National Cancer Institute. Data analysis was performed using SAS Enterprise Guide 7.1 (SAS Institute).
Results
Cohort characteristics
In total, 40 486 individual patients were included in the analysis (Table 2). The median age at diagnosis was 77 years (interquartile range [IQR], 71 to 83 years). The most common NHL subtypes were CLL, diffuse large B-cell lymphoma (DLBCL), and follicular lymphoma (FL). The TRES score was 0 (low risk) in 16 518 patients (40.8%), 1 (intermediate risk) in 14 980 (37.0%), and 2 or 3 (high risk) in 8988 (22.2%). Of the TRES comorbidity categories, vascular comorbidities were the most common, occurring in 15 134 patients (37.4%), followed by upper gastrointestinal comorbidities in 9879 (24.4%), and endocrinal comorbidities in 9565 (23.6%). The most frequent combination of comorbidities in patients with a TRES score of 2 or 3 was a combination of vascular and endocrine comorbidities, which was observed in 3228 patients (8.0%); 1634 patients (4.0%) had comorbidities in all 3 categories. Patients with peripheral T-cell lymphoma and marginal zone lymphoma were more likely to have a TRES score between 2 and 3 than were patients with other subtypes. We also observed that a TRES score between 2 and 3 was more common in older than in younger patients, in men than in women, and in Black and Hispanic patients than in non-Hispanic and White patients. By census tract, patients from regions with higher income and <12 years of education had lower rates of TRES score between 2 and 3 (Table 2).
The most common type of initial treatment was chemoimmunotherapy (30.5% of patients); 38.5% of patient did not receive lymphoma-directed therapy during the study period. The most common first-line regimens were rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (rituximab-CHOP 6041 patients [30.1%]), single-agent CD20 antibody (5327 patients [26.5%]), and bendamustine with rituximab (2664 patients [13.3%]); the most common second-line regimen was single-agent CD20 antibody (3039 patients; 48.9%; supplemental Table 2). On the basis of NHL subtype, the most common first-line regimens were single-agent CD20 antibody in CLL (33.4%), FL (34.8%), lymphoplasmacytic lymphoma (48.8%), and marginal zone lymphoma (62.4%); rituximab-CHOP in DLBCL (63.4%); bendamustine with rituximab in mantle cell lymphoma (42.2%); and CHOP in peripheral T-cell lymphoma (53.2%; supplemental Table 3). TRES score was associated with the likelihood of receiving systemic therapy; in patients with scores of 0, 1, and 2 or 3, rates of receiving first-line systemic therapy were 52.3%, 49.4%, and 45.2%, respectively, and rates of receiving second-line systemic therapy were 17.4%, 15.3%, and 11.8%, respectively (supplemental Table 4). Interestingly, in patients who received treatment, the frequencies of the most common first-line regimens were similar in patients with TRES scores of 0, 1, and 2 or 3 (supplemental Table 5).
High TRES score is associated with increased overall and lymphoma-specific mortality
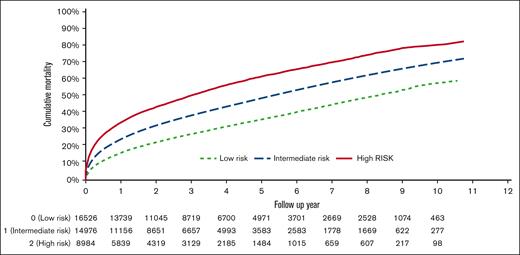
Median follow-up from NHL diagnosis was 33 months (IQR, 13-60). The 3-year cumulative overall mortality rates were 27.6%, 38.8%, and 50.7% for patients with TRES scores of 0, 1, and 2 or 3, respectively. Estimated median OS was 8.2 years, 5.3 years, and 2.9 years in patients with TRES scores of 0, 1, and 2 or 3, respectively (P < .001; Figure 1). In multivariable models, compared with TRES score 0, HRs were 1.27 (95% CI, 1.23-1.31) for TRES score of 1 and 1.67 (95% CI, 1.61-1.74) for TRES score of 2 to 3. Factors associated with high overall mortality included subtype other than CLL, being aged >70 years, being male, being Black, not being married, having stage II-IV NHL, having a previous cancer diagnosis, and lacking NHL treatment (supplemental Table 6). Higher TRES score was associated with increased overall mortality in all NHL subtypes (Table 3; supplemental Figure 1).
The cause of death was reported for 30 155 patients (cause of death was not available in all SEER registries), among whom TRES score was 0 in 41.6%, 1 in 36.7%, and 2 to 3 in 21.6%. The most common cause of death was NHL (7110 patients; 23.6%), followed by non-NHL cancer (5183; 17.2%) and other causes (2180; 7.2%).
The 3-year cumulative NHL-specific mortality rates were 16.6%, 22.0%, and 27.1% for patients with TRES scores of 0, 1, and 2 or 3, respectively (P < .001; Figure 2A). Higher TRES scores were associated with increased NHL-specific mortality in multivariable models; HRs were 1.10 (95% CI, 1.04-1.16) for TRES score of 1 vs 0 and 1.23 (95% CI, 1.15-1.31) for TRES score of 2 or 3 vs 0. Factors associated with increased risk of NHL-specific mortality included subtypes other than CLL, being aged >70 years, being male, not being married, having stage II, III, or IV NHL, and lacking NHL treatment (supplemental Table 6). Higher TRES score was associated with increased NHL-specific mortality in DLBCL, FL, CLL, and cutaneous T-cell lymphoma (Table 3).
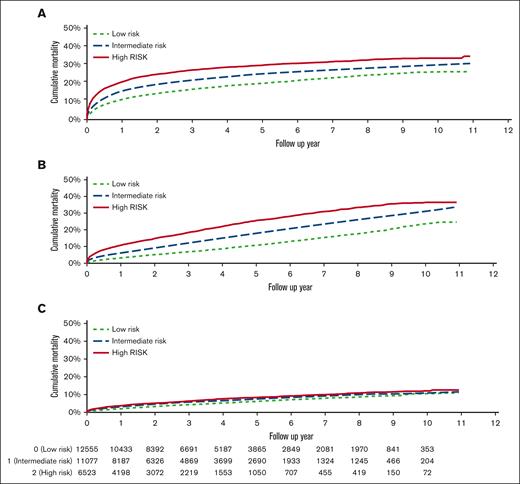
Cause specific mortality by TRES score. Mortality due to (A) NHL, (B) non-NHL cancer, and (C) noncancer causes based on TRES score.
Cause specific mortality by TRES score. Mortality due to (A) NHL, (B) non-NHL cancer, and (C) noncancer causes based on TRES score.
To control for differences in treatment frequency between TRES score groups, we performed a propensity-matched analysis of OS and NHL-specific survival. The probability of receiving NHL-directed therapy was assessed independently in all NHL subtypes and included in multivariable models. We found that TRES score remained independently associated with OS in all evaluated NHL subtypes and with NHL-specific survival in DLBCL, FL, and CLL (Table 4).
We also analyzed death due to cancers other than NHL. The 3-year cumulative mortality rates were 6.9%, 12.0%, and 18.4% for patients with TRES scores of 0, 1, and 2 or 3, respectively (P < .001; Figure 2B). High TRES scores were associated with increased non-NHL cancer mortality in multivariable models; compared with a TRES score of 0, HRs were 1.41 (95% CI, 1.32-1.51) for TRES score of 1 and 1.94 (95% CI, 1.80-2.08) for TRES score of 2 or 3. Other variables associated with non-NHL cancer mortality were subtype, stage, age, sex, marital status, and initial treatment (supplemental Table 6). Higher TRES score was associated with increased non-NHL cancer mortality in all NHL subtypes except peripheral T-cell lymphoma (supplemental Table 6). Surprisingly, death due to nonmalignant causes, most frequently heart diseases, was similar across TRES score groups (log-rank test, P < .001; Figure 2C). We also evaluated the association of TRES score and infection related mortality. Three-year infection related mortality was 0.8%, 1.0%, and 1.4% in patients with TRES scores of 0, 1 and 2 or 3, respectively (P = .001).
Discussion
TRES was previously developed and validated among patients with CLL with the goal of providing a simple-to-use comorbidity scale for patients with CLL. This study aims to assess the TRES score in patients with other NHL subtypes with the ultimate goal of developing a clinical score that could standardize the assessment of comorbidities among patients with NHL, be implemented in clinical practice, and be used as a research tool to improve outcomes in patients with NHL and comorbidities, a population that is frequently excluded from clinical trials and has not derived an equal benefit from recent advances in NHL treatment.2,3,12,23 In this study, we found that TRES score was associated with OS, lymphoma-specific, and more broadly cancer-specific survival in patients with NHL. Previously, TRES had been studied in 3 independent cohorts of patients with CLL (totaling ∼5700 patients), in which it had been shown to be associated with OS and event-free survival.16,17 Notably, the initial cohort of patients with CLL who expected to develop the TRES score were treated with either ibrutinib (59%) or CD20 antibody with or without chemotherapy, which is distinct from the treatment of patients with NHL included in this study.16 To the best of our knowledge, this study is the first to demonstrate an association between TRES score and survival in NHL subtypes other than CLL.
In this study, median OS was 8.2, 5.3, and 2.9 years in patients with TRES scores of 0, 1, and 2 or 3, respectively, demonstrating that TRES score is associated with clinically meaningful differences in survival of patient with NHL. Similar differences in OS were observed in all evaluated NHL subtypes. In models adjusted for NHL subtype, treatment, age, and other demographic factors, TRES remained independently associated with OS. Patients with a TRES score of 1 (intermediate) had a 27% increased risk of death, and those with a score of 2 or 3 (high) had a 68% increased risk of death compared with the patients with a score of 0 (low).
We also found that TRES score was associated with lymphoma-specific survival and remained independently associated with lymphoma-specific survival in adjusted models. Patients with a TRES score of 1 were 10% more likely to die of NHL, and those with a TRES score of 2 or 3 were 24% more likely to die of NHL compared with the patients with a TRES score of 0. In addition, propensity-weighted matching TRES score remained independently associated with lymphoma-specific survival. This is a strength of TRES because previously evaluated methods to assess comorbidities in patients with NHL have shown differences in OS but have produced inconsistent results regarding lymphoma-specific survival.6,24,25 In this cohort, 49% of patients died of lymphoma, and 85% of deaths were attributable to cancer. Interestingly, we noted that TRES was not predictive of noncancer mortality.
Through the ongoing work, our group aims to assess the use of the TRES score to guide treatment and ultimately improve survival for this population who are at risk. In this regard, TRES has several distinct advantages compared with other comorbidity scales used in NHL clinical research, such as cumulative illness rating scale,15 Charlson comorbidity index,26 and hematopoietic cell transplantation–specific comorbidity index7 and compared with geriatric assessment tools that incorporate comorbidities into the assessment of patients with NHL.4,8 Firstly, TRES has 3 categories, compared with the 14 to 16 categories of the other scales, which makes TRES more easily adaptable to clinical practice. Secondly, TRES may provide greater insights into the mechanistic underpinnings that link comorbidities with worse outcomes in patients with NHL by identifying the most important prognostic categories rather than all possible medical comorbidities. In addition, we have shown that in patients with CLL, TRES score is associated with T-cell phenotype and physical fitness.27 Finally, TRES was developed specifically for patients with CLL, rather than the general medicine populations used in the development of other scales.
Patients with NHL who are at high risk should be the focus of prospective clinical trials. Clinical prognostic tools currently in use, such as the international prognostic index (IPI), which have been used to recruit high-risk patients for clinical trials,28,29 do not include a measure of comorbidity.30-32 Our previous work in CLL demonstrated that TRES is independently associated with OS when adjusted for CLL IPI score, suggesting that combining TRES with other models may improve the identification of high-risk patients.17 We were unable to perform these analyses in this study because, unfortunately, the SEER-Medicare database does not include performance status or lab values which are used in NHL prognostic scores, such as the IPI. In addition, SEER-Medicare does not include molecular subtyping or mutational analyses so these variables could not be included in the models presented in this manuscript.
There are limitations inherent to the use of large national cancer registries for clinical research. Although the SEER-Medicare database does have extensive information regarding cancer diagnosis, patient demographics, and death, it does not include laboratory or molecular information. In addition, our study did not include patients aged <66 years, potentially limiting the generalizability of our findings to younger patients. This may be particularly relevant to the study of comorbidities because both total comorbidity burden and the proportion of severe comorbidities are higher in older patients with CLL.2,3,23 However, the initial cohorts used to develop and validate TRES in CLL included patients aged ≤65 years, which accounted for 48% of patients in the initial discovery cohort and 53% in the validation cohort.16 Through the ongoing work our group aims to validate these findings, in an NHL subtype specific manner, using independent datasets that include younger patients.33 Further validation of this score in NHL subtypes other than CLL should be pursued before incorporation into prospective studies.
Here, we demonstrate that a simple-to-use comorbidity scale previously developed in patients with CLL is broadly applicable to older patients with NHL. The TRES score is independently associated with OS and lymphoma-specific survival. TRES can typically be completed in <1 minute and can be applied in resource-limited settings. Future development of TRES aims at assessing its use as a predictive marker that could guide treatment and clinical research for patients with comorbidities and NHL or other cancers.
Acknowledgments
The authors thank Stephanie Deming, Research Medical Library, MD Anderson Cancer Center, for editing the manuscript. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services Inc; and the SEER program tumor registries in the creation of the SEER-Medicare database. This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors.
M.J.G is supported by a T32 training grant (5T32CA009666-27). S.H.G is supported by CPRIT RP160674 and Komen SC150061. A.V.D is an LLS Clinical Scholar (#2319-19) and S.H.G is also partially supported by Cancer Center Support Grant NCI P30 CA016672.
Authorship
Contribution: M.J.G., A.V.D., and S.H.G. conceptualized the study, directed the analysis, and wrote the manuscript; Z.D. and H.Z. developed the statistical methodology, performed the analysis, and wrote the manuscript; L.N. and A.F. wrote and reviewed the manuscript; A.V.D. and S.H.G. supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sharon H. Giordano, Colin Powell Chair for Cancer Research, Chair, Department of Health Services Research, The University of Texas MD Anderson Cancer Center, 1400 Pressler St, Unit 1444, Houston, TX 77030; e-mail: sgiordan@mdanderson.org.
References
Author notes
Presented in abstract form at the 64th annual meeting of the American Society of Hematology, Atlanta, GA, 12 December 2022.
Data are available on request from the corresponding author, Sharon H. Giordano (sgiordan@mdanderson.org).
The full-text version of this article contains a data supplement.