TO THE EDITOR:
Autologous stem cell transplant (ASCT) is currently the standard consolidative approach for fit patients with large B-cell lymphoma (LBCL) who achieve a complete response (CR) after salvage therapy, whereas autologous anti-CD19 chimeric antigen receptor T-cell therapy (CART) is commonly reserved for those who are chemo-refractory or ASCT-ineligible.1-6 However, the optimal management for patients who achieve a partial response (PR) remains debated. In a recently published retrospective registry study, 222 patients with LBCL who received ASCT in PR were compared with 126 patients in PR who received CART, with a significantly lower relapse/progression rate observed in the former.7 However, in addition to including patients who were not restaged after bridging therapy, the study did not account for differences in baseline tumor burden before ASCT or CART, because the total metabolic tumor volume (TMTV) may significantly vary among patients with PR, and prior studies have revealed that high TMTVs can negatively affect the patient’s response to CART.8
This is a single-center retrospective study of all patients with relapsed and/or refractory LBCL who achieved a PR based on the Lugano 2014 classification and then underwent either ASCT between 2010 and 2021 or CART with axicabtagene ciloleucel between 2018 and 2022.9 Patients who previously received ≥2 lines of systemic therapy were included in the study. Refractory disease was defined as a lack of response to any prior line of systemic therapy before the latest documented PR. Patients without restaging positron emission tomography scans after bridging therapy were excluded; patients who had previously received CART (for the ASCT cohort) or ASCT (for the CART cohort) were also excluded. The study was approved by The University of Texas MD Anderson Cancer Center institutional review board and was conducted in accordance with the institutional guidelines and principles of the Declaration of Helsinki. The clinical characteristics and laboratory features were collected before the initiation of conditioning and lymphodepleting chemotherapy. TMTV was calculated as previously described.8
The primary end point was a 24-month relapse/progression rate, defined as the time from infusion to relapse or disease progression. The secondary end points included overall survival and the identification of factors associated with TMTV. Survival curves were calculated using Kaplan-Meier estimates, and the cumulative incidence function with Gray test for subdistribution hazards was used to compare the relapse/progression rates in the 2 cohorts to account for competing events. The Fine-Gray model was used for multivariate analysis of factors significantly associated with the relapse/progression rate on univariate analysis. The median follow-up for all patients was calculated using the inverse Kaplan-Meier method. For the whole population, the difference in TMTV, measured as a continuous variable, between patient groups was evaluated using the Mann-Whitney test. To adjust for differences in baseline characteristics, propensity score matching analysis was used with a 1:1 ratio. P ≤ .05 (two-tailed) was considered statistically significant. Statistical analyses were performed using SAS 9.4, SPSS 24, and GraphPad Prism 8.
A total of 111 patients with relapsed or refractory LBCL in PR after the latest line of therapy were included in the study, 69 of whom underwent ASCT and 42 underwent CART. None of the patients had previously received allogeneic stem cell transplantation. Patients who underwent CART more frequently had advanced stage disease (81% vs 32%; P < .001), >1 extranodal site of disease (45% vs 7%; P < .001), elevated International Prognostic Index (IPI; 40% vs 20%; P = .03), refractory disease (71% vs 28%; P < .001), and received >2 lines of systemic therapy more frequently (86% vs 39%; P < .001). The remaining baseline characteristics of the patients are listed in Table 1.
The median follow-up was 38 months (95% confidence interval, 26-49 months), which was significantly longer for patients who received ASCT (76 vs 26 months; P = .001). Upon univariate analysis, despite an enrichment in adverse prognostic factors, the relapse/progression rate was 44% for patients who received CART compared with 59% for those who received ASCT (P = .48). After performing propensity score matching, including IPI, refractory status, and number of previous lines of systemic therapy, 2 matched cohorts of 26 patients each were identified. When comparing these 2 groups, the relapse/progression rate was 40% for patients who received CART and 58% for those who received ASCT (P = .53).
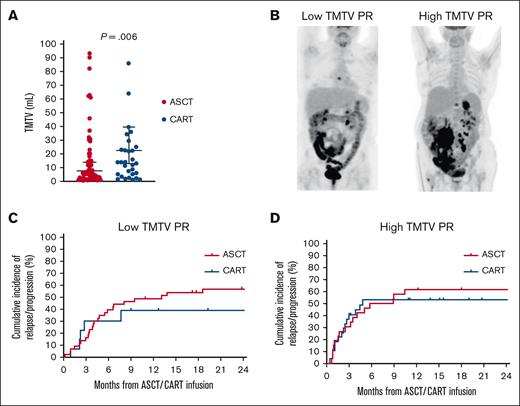
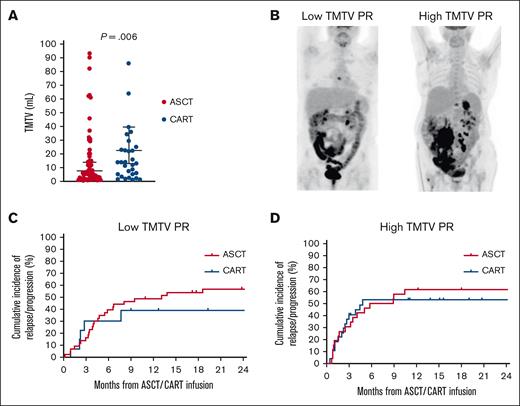
TMTV was measured in all patients with a median of 12 mL (range, 0.85-847 mL). TMTV was significantly higher in patients who received CART than in those who received ASCT (23 vs 8 mL; P = .006; Figure 1A). The factors associated with high TMTV were low European Cooperative Oncology Group performance status (P = .007), advanced stage (P < .001), high number of extranodal sites (P = .002), and high IPI (P = .002). Patients were subsequently categorized as having low (n = 57) or high (n = 54) TMTV based on the median value (Figure 1B). Among patients with low TMTV, the relapse/progression rate was 43% for patients who received CART compared with 61% for those who received ASCT (P = .49; Figure 1C). In contrast, among patients with high TMTV, relapse/progression rates were similar between the 2 groups (P = .98), as a consequence of an increased rate among those who received CART (53%) and unchanged among those who received ASCT (62%; Figure 1D). Subsequently, TMTV was divided into quartiles (0.85-3.9, 4-12, 13.4-37, and 39.6-847), and a progressively smaller hazard ratio for relapse/progression rate was observed in quartiles from 1 to 4 (1.63, 1.47, 1.31, and 1.30, respectively) when comparing CART with ASCT. In a multivariate analysis that included TMTV, IPI, and the number of previous therapies, only TMTV maintained its association with relapse/progression rate (hazard ratio, 2.2; 95% confidence interval, 1.1-5; P = .049).
Cumulative incidence of relapse/progression according to TMTV. (A) Distribution of the TMTV in patients who underwent ASCT or CART. (B) Example of low and high TMTV PR. (C) Cumulative incidence of relapse/progression in patients with a low TMTV PR. (D) Cumulative incidence of relapse/progression in patients with a high TMTV PR.
Cumulative incidence of relapse/progression according to TMTV. (A) Distribution of the TMTV in patients who underwent ASCT or CART. (B) Example of low and high TMTV PR. (C) Cumulative incidence of relapse/progression in patients with a low TMTV PR. (D) Cumulative incidence of relapse/progression in patients with a high TMTV PR.
The median overall survival was 9 months for patients who received ASCT and was not reached for those who received CART.
In this study, we demonstrated that tumor burden, as measured by TMTV, differentially affects the response to CART and ASCT among patients with LBCL who achieve PR. In fact, no change in the relapse/progression rate was observed among patients with PR who proceeded to ASCT based on TMTV, but it proportionally increased corresponding to the TMTV among those who proceeded to receiving CART.
Multiple studies have previously shown that low tumor burden is associated with improved outcomes after CART, with excellent responses observed in patients able to achieve CR after bridging therapy.8,10-12 The biological mechanism underlying this phenomenon seems to be related to the immunosuppressive effect exerted by intratumoral macrophages, which are enriched in patients with high tumor burden. In fact, higher numbers of tissue macrophages with upregulated interferon signaling were associated with lower peak CAR T-cell expansion and worse outcomes in patients with LBCL treated with CART.13,14 In addition, polymorphisms in genes crucial for macrophage activation correlated with suboptimal response to conditioning chemotherapy, and gene signatures characteristic of protumoral macrophages were enriched in patients who did not achieve CR after CART.15,16 Response to ASCT is instead mainly mediated by chemo-sensitivity, likely explaining the lack of impact of tumor burden on its efficacy among patients with LBCL in PR.17
In our analysis, elevated IPI and its components were associated with high TMTV. Although pretreatment IPI was not available in the aforementioned retrospective study, patients who received CART had a significantly higher rate of bulky disease than those who received ASCT while in PR.7 This suggests that high TMTV PRs may have been overrepresented among patients who received CART in this analysis, which likely explains their inferior outcomes. Moreover, in the same study, the difference in the relapse/progression rate was not maintained after matched propensity score analysis. The current criteria to assess the response to treatment in lymphoma do not account for the heterogeneity of PR, and their integration with TMTV may help better stratify patients and better predict subsequent outcomes.
We acknowledge the multiple limitations of our study, including its single center and retrospective nature and the difference in supportive care available during the different timeframes when ASCT and CART were performed.
In conclusion, for patients with LBCL in PR at the time of treatment, TMTV has a greater impact on outcomes after CART than after ASCT. Analyses comparing these 2 strategies should consider this potential bias. Although larger and prospective studies are needed to confirm these findings, our results serve as a reminder that not all PRs are equal and that CART remains a viable option for patients with low TMTV PR.
Acknowledgments: This research is partially supported by The University of Texas MD Anderson Cancer Center Support Grant from the National Institutes of Health, National Cancer Institute (P30 CA016672).
P.S. is supported by the Lymphoma Research Foundation Career Development Award, the Leukemia Lymphoma Society Scholar in Clinical Research Career Development Program, the Sabin Fellowship Award, and R21 National Institutes of Health grant.
Contribution: P.S., O.P., E.J.S., and S.A. designed the study, analyzed the data, provided clinical care to patients, and wrote the manuscript; G.X. and S.O.T. measured the TMTV in all patients; L.F. provided statistical support and wrote the manuscript; A.A.Z., A.J., S.S.N., and P.K. provided clinical care to patients and wrote the manuscript; and J.V., K.O., and M.S. collected clinical data, provided clinical care to patients, and wrote the manuscript.
Conflict-of-interest disclosure: P.S. is a consultant for Roche-Genentech, Kite-Gilead, Hutchison MediPharma, AstraZeneca-Acerta, ADC Therapeutics, Sobi, and TG Therapeutics, and has received research funds from Sobi, AstraZeneca-Acerta, ALX Oncology, and ADC Therapeutics. S.A. has research support to institution for clinical trials from Seattle Genetics, Merck, Xencor, Chimagen, and Tessa Therapeutics; has membership on Tessa Therapeutic’s and Chimagen’s scientific advisory committee; serves on the data safety monitoring board for Myeloid Therapeutics; and is a consultant for ADC Therapeutics and Kite-Gilead. S.O.T. holds private stocks in Starton Therapeutics, CoapTech LLC, Lutroo Imaging, NeuroMedica, Beyond Physician, and Xense Inc. The remaining authors declare no competing financial interests.
Correspondence: Paolo Strati, Division of Cancer Medicine, Department of Lymphoma and Myeloma, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 429, Houston, TX 77030; e-mail: pstrati@mdanderson.org.
References
Author notes
∗O.P., E.J.S., and S.A. contributed equally to this study.
Data are available on request from the corresponding author, Paolo Strati (pstrati@mdanderson.org).