Key Points
Primary graft failure may be identified by early chimerism analysis after DUCBT with RIC.
Chimerism does not appear to be useful in predicting relapse.
Abstract
Umbilical cord blood transplantation (UCBT) has increased access to potentially curative therapy for patients with life-threatening disorders of the bone marrow and immune system. The introduction of reduced intensity conditioning (RIC) regimens and double umbilical cord unit infusions (DUCBT) has broadened the applicability of UCBT to more frail or larger recipients. The kinetics of chimerism after RIC DUCBT and their clinical utility are poorly understood. The RIC CBT trial reported here sought to prospectively evaluate the role of lineage-specific chimerism after DUCBT in adult patients with hematologic malignancies in the United Kingdom. Fifty-eight patients with a median age of 52 years were recruited, with overall and progression-free survivals of 59% (95% confidence interval [CI], 45%-71%) and 52% (95% CI, 39%-64%), respectively, at 2 years. Nonrelapse mortality was 4% (95% CI, 1%-13%) at day 100, and the relapse rate was 31% (95% CI, 21%-45%) at 1 year. Peripheral blood lineage-specific chimerism was feasible from day 7 after transplant onward. Five patterns of chimerism were observed including (1) complete single unit dominance (39 patients), (2) sustained donor-donor mixed chimerism (3 patients), (3) sustained donor-recipient mixed chimerism (5 patients), (4) dominance reversion (1 patient), and (5) primary graft failure (4 patients). The RIC CBT trial enabled adult patients with high-risk hematologic malignancies to safely access UCBT in the United Kingdom and provided novel insights into the kinetics of donor and recipient chimerism after RIC DUCBT that are clinically relevant. This trial was registered at https://www.clinicaltrialsregister.eu/ctr-search/trial/2004-003845-41/GB as #NCT00959231 and EudraCT 2004-003845-41.
Introduction
Over the last two decades, umbilical cord blood (UCB) has become an established alternative source of haemopoietic stem cells. This has led to potentially curative allogeneic transplantation being accessed by thousands of patients with diseases of the bone marrow and immune system who would otherwise have been precluded for lack of a suitably HLA matched sibling or adult volunteer unrelated donor.1,2
The early success reported in children was initially not replicated in adults because of the lower progenitor cell dose per body weight of UCB, leading to poorer engraftment and excess transplant-related mortality (TRM), and the increased toxicity of ablative regimens in older patients or those with multiple comorbidities.3
The use of reduced intensity conditioning (RIC) regimens4 combined with strategies to augment cell dose have broadened the applicability of UCB transplantation to larger adolescents and adults. One of the most effective approaches to increase cell dose has been the coinfusion of 2 UCB units (double umbilical cord blood transplants [DUCBT]), a strategy pioneered at the University of Minnesota.1,5
Although DUCBT is thought to promote engraftment5 with both units contributing to early hematopoiesis, in more than 90% of patients, only 1 UCB unit will ultimately predominate and exclusively provide long-term hematopoiesis.1,6-8 However, the factors predicting this, the kinetics of engraftment, and the physiology responsible for this observation are poorly understood.
A better understanding of the kinetics of engraftment is potentially important in predicting which cord blood unit (CBU) will become dominant, better selection of CBUs, early prediction of graft failure, understanding of the mechanisms of graft-versus-leukemia and graft-versus-host disease (GVHD), and how DUCBT could potentially provide a platform for posttransplant immune modulation.
This study aimed to investigate the patterns and clinical relevance of early and long-term chimerism in patients recruited to the phase 2 RIC UCBT trial (transplantation of umbilical cord blood from unrelated donors in patients with hematologic diseases using a RIC regimen).
Methods
Study design and participants
The RIC UCBT trial was a phase 2 trial, recruiting patients from 15 UK transplant centers. Patients were eligible for the trial if they met the following 4 inclusion criteria: (1) were 2 to 70 years of age, (2) had a high-risk hematologic malignancy for which an allogeneic hematopoietic stem cell transplant was indicated, (3) had no HLA matched (10/10 allelic) sibling or unrelated donor available, and (4) were not eligible for an ablative conditioning regimen because of older age or comorbidities. All participants gave written informed consent, and the trial was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the Riverside Ethics Committee (08/H0706/92) and Medicines and Healthcare Products Regulatory Agency (21786/0203/001).
Procedures.
CBU selection was based on cryopreserved total nucleated cell (TNC) dose and HLA-A, -B, and -DRB1 match (HLA-A and -B matching at antigen level and HLA-DRB1 at allelic level). All units were ≥4/6 matched to the recipient with no requirement for interunit matching. A single unit was used for transplantation if the TNC ≥ 3.0 × 107/kg for a 6/6 match or TNC ≥ 4.0 × 107/kg for a 5/6 match. If no adequate single unit was available, a double unit graft was selected, in which each unit had a TNC ≥ 1.5 × 107/kg. An expert national unit selection committee was established to facilitate optimum unit selection.
All patients received a RIC regimen comprised of fludarabine 40 mg/m2 per day on days −6 to −2 (total 200 mg/m2), cyclophosphamide 50 mg/kg on day −6, and total body irradiation 200 cGy on day −1. No patients received serotherapy. All patients received granulocyte colony-stimulating factor (lenograstim) 5 µg/kg between day +7 until absolute neutrophil count was >2.5 × 109/L for 2 consecutive days. GVHD prophylaxis was mycophenolate mofetil from days −3 to +35 or 7 days after engraftment (whichever was later), followed by taper and cyclosporine on days −3 to +100, followed by taper if no GVHD.
The end of the study was defined as the time when the final patient had been followed up for 2 years after transplant. Nonrelapse mortality (NRM) at 100 days after transplant was defined as the time from infusion to death not caused by relapse. Patients who relapsed were censored at the date of relapse and patients who did not relapse and did not die were censored at the date of last follow-up. Overall survival was the time from infusion until a patient died of any cause. Surviving patients were censored at the date of last follow-up. Relapse-free survival was the time from infusion until a patient relapsed or died, whichever occurred first. Patients who did not relapse and did not die were censored at the date last of last follow-up. Time to relapse was the time from infusion until a patient relapsed. Patients who did not relapse were censored at the date of last follow-up or date of death.
Hematopoietic recovery was defined as (1) time to first of 3 consecutive days with absolute neutrophil count > 0.5 × 109/L after first posttransplant nadir, (2) time to platelets > 20 × 109/L (first of 3 consecutive days) with no platelet transfusions in the 7 preceding days, and (3) time to red blood cell independence (hemoglobin > 9 g and no transfusions for 15 days). Patients who did not recover by day 100 were censored at day 100, and patients who recovered after day 42 but a date was not provided were censored at day 42.
Lineage-specific chimerism studies (peripheral blood mononuclear cells [PBMCs], B cells, T cells, and granulocytes) were performed according to local laboratory procedures on 5 to 10 mL EDTA peripheral blood samples on days 7, 14, 21, 28, 35, 60, and 100, 6 months, and 1 and 2 years to determine the relative contribution of donor and recipient to overall hematopoiesis.
Primary engraftment was defined as neutrophil recovery associated with detectable donor chimerism within the first month after transplantation. Sustained donor engraftment was defined as ongoing neutrophil recovery and donor hematopoiesis beyond day 42. Primary graft failure (GF) was defined as failure to achieve sustained donor engraftment. Complete donor chimerism was defined as marrow reconstitution of at least 90% donor origin.
Adverse events and cord blood infusion-associated complications were reported using Common Terminology Criteria for Adverse Events v4.0. Bacterial, fungal, parasitic, and viral infections and the incidence of acute and chronic GVHD were reported separately.
Data analysis
Continuous variables were summarized as median or mean and range and categorical variables as frequency and percentages. Time to event outcomes were summarized in terms of event or event-free rates at prespecified timepoints and plotted using the Kaplan-Meier method. The time from transplant to neutrophil recovery, platelet recovery, and red blood cell recovery in days was also summarized using Kaplan-Meier methods (median time to recovery and rates of recovery at 42 and 100 days after transplant).
Frequencies and percentages were used to summarize the maximum severity of adverse events (>grade 3), the occurrence of cord blood infusion-associated complications, GVHD, and infections.
The association between intrinsic cord blood unit variables and chimerism outcome was measured using univariate multilevel logistic regression, which was fitted by considering each patient a random intercept. A paired t test was also performed to assess differences between the dominant and nondominant unit and the effect of time in the change of the % donor PBMCs, B cells, T cells, and granulocytes. The mean and range of the chimerism between patients with primary graft failure were compared with those engrafting.
Results
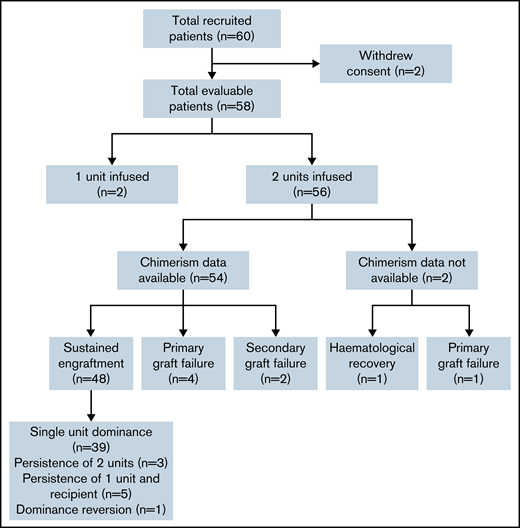
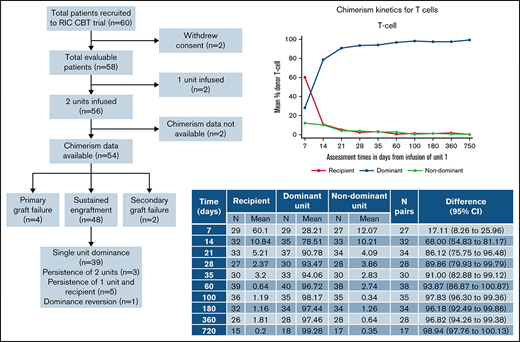
The target accrual was achieved, with 60 patients recruited between December 2009 to February 2014 (Figure 1). Two patients withdrew consent from trial participation and were removed from all analyses. Of 58 evaluable patients, the median follow-up was 48.7 months (95% confidence interval [CI], 38-60 months). Patient characteristics are shown in Table 1: recipients were predominantly male (n = 35; 60%), were White (n = 40; 69%), had a Karnofsky performance status of 90% to 100% in 51 patients (88%), had a comorbidity index of 0 to 1 in 40 patients (69%), and had a median age of 52 years (range, 20-68 years). The indication for transplantation was acute leukemia or myelodysplasia in 71% of patients (acute myeloid leukemia, 47%; acute lymphoblastic leukemia, 12%; myelodysplasia, 12%) with 40 (69%) of patients having 2 or more lines of previous therapy. All patients received the conditioning regimen and GVHD prophylaxis according to protocol. Two patients had a sufficient single CBU graft, as defined in the trial cord selection algorithm (these patients were 26 and 65 years of age, with weights of 76.5 and 45.4 kg, respectively), with the remaining 56 patients receiving 2 CBUs.
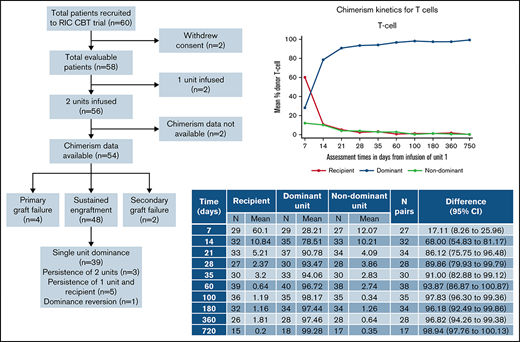
Consort diagram showing recruitment, chimerism analysis availability, and engraftment/chimerism outcomes.
Consort diagram showing recruitment, chimerism analysis availability, and engraftment/chimerism outcomes.
Time to event outcomes
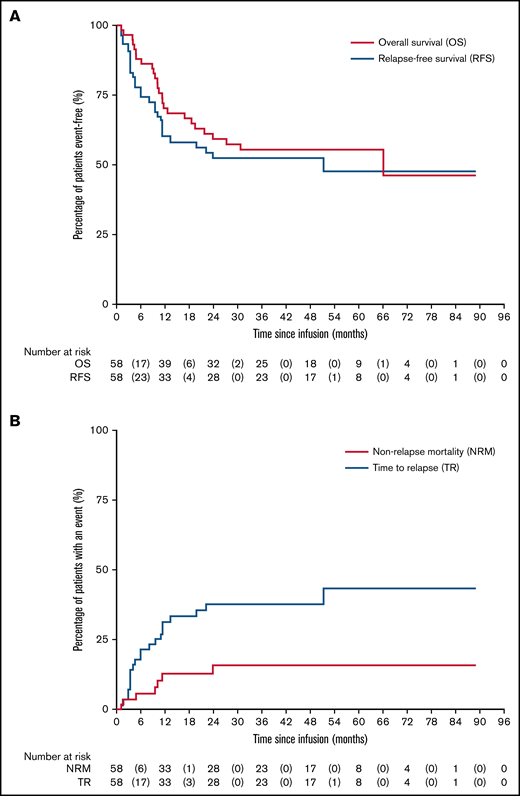
Figure 2 depicts time to event outcomes. Overall survival was 97% (95% CI, 87%-99%) at 3 months, 70% (95% CI, 57%-80%) at 1 year, and 59% (95% CI, 45%-71%) at 2 years, with a progression-free survival of 90% (95% CI, 79%-95%) at 3 months, 60% (95% CI, 46%-71%) at 1 year, and 52% (95% CI, 39%-64%) at 2 years. The NRM was 4% (95% CI, 1%-13%) at day 100, and the relapse rate was 31% (95% CI, 21%-45%) at 1 year. The cause of death was relapse or disease progression in 17 patients, transplant related in 8 patients, and unknown cause of death in 1 patient.
Clinical outcomes following RIC CBT. (A) Overall and relapse-free survivals. (B) NRM and time to relapse.
Clinical outcomes following RIC CBT. (A) Overall and relapse-free survivals. (B) NRM and time to relapse.
The overall incidence of grade II to IV acute GVHD was 33% (19 patients) and III to IV acute GVHD was 24% (14 patients). By the completion of the study, 12 patients (21%) experienced limited chronic GVHD and only 2 (3%) had extensive chronic GVHD. A detailed summary of reported adverse events, specific infections observed, and cord blood infusion reactions are given in supplemental Tables 1 to 3.
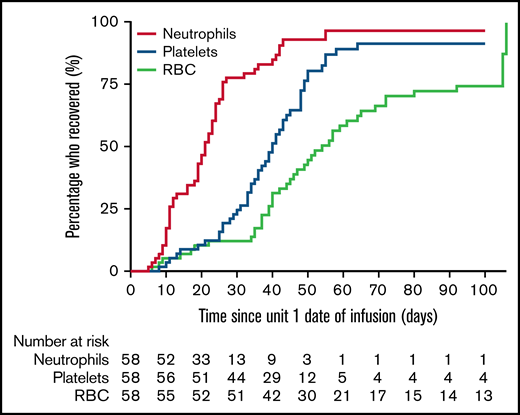
Hematopoietic recovery is presented in Figure 3. Neutrophil recovery was observed at a median time of 21 days (range, 5-55 days), with 52 (90%) patients achieving neutrophil recovery by day 42. The median time to platelet recovery was 38 days (range, 8-64 days), with 49 (84%) patients achieving platelet recovery by day 100. Red cell recovery was observed at a median of 51 days (range, 6-106 days), with 40 (69%) achieving red cell independence by day 100. Primary graft failure was observed in 5 patients, and 2 patients had secondary graft failure.
Chimerism analyses
Peripheral blood chimerism analysis was available for 54 of the 56 patients having a double UCBT, of whom 4 had primary graft failure and 2 secondary graft failure (Figure 1). Chimerism data were reliably available at all time points for PBMC, T cells, and granulocytes, although B-cell chimerism was less frequently reported.
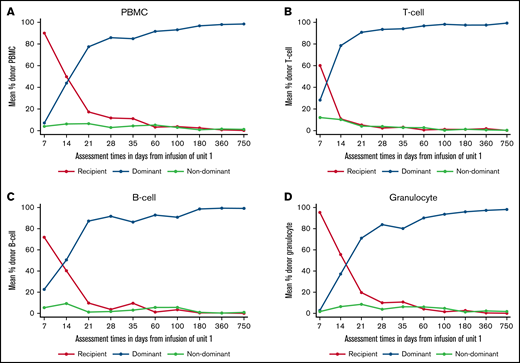
In the 48 patients who engrafted after DUCBT and for whom chimerism results were available, 5 different patterns of chimerism kinetics were observed: (1) early contribution of both units and recipient but eventual complete dominance of a single UCB unit with disappearance of the second unit and recipient (39 patients; Figures 4A-D), (2) sustained donor-donor chimerism with an ongoing contribution of both units and loss of recipient (3 patients), (3) sustained donor-recipient mixed chimerism with contribution of recipient and 1 of the units (5 patients), (4) dominance reversion (1 patient), and (5) primary graft failure (5 patients).
Chimerism kinetics following RIC CBT. (A) Chimerism kinetics for PBMCs. (B) Chimerism kinetics for T cells. (C) Chimerism kinetics for B cells. (D) Chimerism kinetics for granulocytes.
Chimerism kinetics following RIC CBT. (A) Chimerism kinetics for PBMCs. (B) Chimerism kinetics for T cells. (C) Chimerism kinetics for B cells. (D) Chimerism kinetics for granulocytes.
For the engrafting patients, the recipient contribution to all lineages decreased rapidly and was no longer detectable by days 28 to 35. In the first 7 to 21 days after transplant, both cord blood units were detectable in all lineages. However, the dominant unit increased rapidly and was the only contribution to hemopoiesis in most patients by days 28 to 35. The dominant unit could be identified by day 7 in the T-cell and B-cell lineages and by day 14 in PBMC and granulocytes.
In the 3 patients with persistence of both cord units, the inter-unit HLA match was 4/6 for 1 patient and 6/6 for 2 patients. None of these 3 patients relapsed. In the 5 patients who engrafted with persistent donor-recipient mixed chimerism, the HLA match between patient and the persisting unit was 4/6 for 4 patients and 5/6 for 1 patient. The HLA match between the nonpersisting unit and the recipient was 4/6 in 1 patient, 5/6 in 2 patients, 6/6 in 1 patient, and not known in 1 patient. One of these 5 patients relapsed. In the 1 patient in whom dominance reversion was observed, the recipient contribution decreased rapidly as with other patients. However, 1 unit appeared to be increasing in the first 2 to 4 weeks after transplant but then decreased, whereas the second unit subsequently increased and became the persisting dominant unit, which was solely responsible for sustained hematopoiesis. The HLA match between each unit was 5/6 for unit 1 and 4/6 for unit 2 (the interunit matching is unknown), and this patient subsequently relapsed.
Chimerism and relapse
Of the 48 patients who did not have graft failure and for whom chimerism results are available, once stable donor chimerism was achieved, this was sustained for the duration of follow-up for 41 patients (85%). For the remaining 7 patients, recipient chimerism subsequently became detectable, and 6 of these patients relapsed (all patients had acute myeloid leukemia). Of the 21 patients who relapsed overall, 15 had stable chimerism before relapse, including 11 patients with acute leukemia, 2 with Hodgkin lymphoma, 1 with follicular lymphoma, and 1 with multiple myeloma.
Chimerism and GF
Table 2 summarizes the chimerism kinetics between patients who experienced GF compared with those who engrafted. Five patients experienced primary GF, and chimerism data were available for 4 of these. The small number of patients precludes testing for statistical significance of the clear differences observed. However, no patient with primary GF had a combined CBU chimerism in PBMC or T cells of >10% from day 21 onward, and no engrafting patients had <10% combined donor chimerism at these time points. A donor contribution to granulopoiesis was not detectable from day 21 onward in those with GF and was always present in engrafting patients. B-cell chimerism results were only available for 1 patient with GF.
Association between intrinsic UCB variables and the dominant unit
Table 3 summarizes the association between pretransplant CBU characteristics in determining which unit became dominant or nondominant. On multivariate analysis, only the order of unit infusion was significantly associated with unit dominance, with the first unit infused more likely to be dominant.
Discussion
This is the first study to prospectively describe both early (before day 21) and late lineage-specific chimerism data in DUCBT after the most commonly used RIC regimen (fludarabine, cyclophosphamide, and low-dose total body irradiation) and is the only study to report chimerism kinetics in primary GF in this context (Table 4). The clinical outcomes in this phase 2 trial compare favorably to other reported series.4,9 Despite the low white cell count in the immediate post-DUCBT period, lineage-specific chimerism was feasible using peripheral blood samples at all time points studied.
In 81% of patients achieving sustained donor engraftment in our study, a single UCB became the dominant contributor to hematopoiesis with eradication of the second UCB unit and residual recipient bone marrow, usually within the first 35 days after transplant. This emergence of single CBU dominance is well described after DUCBT, appears to be slower after RIC compared with myeloablative conditioned DUCBT (Table 4), and is postulated to be caused by intrinsic factors of the CBUs, immune-mediated reactions between both cord units and between each unit and recipient, recipient bone marrow microenvironment, and different homing efficiencies.6
The only variable found to be predictive of the dominant CBU in our study was order of infusion, with the first infused unit being more likely to persist. Two other studies have similarly reported that units infused between 3.5 and 4.5 hours before the second were more likely to become dominant.10,11 Ballen et al10 postulate that the first infused cells have a competitive advantage in populating a limited capacity within the stem cell niche. A range of other intrinsic factors have been reported to predict CBU dominance including CD8+ T-cell dose,12 CD3+-cell dose,5,6,8,13,14 naïve CD3+CD8+ subset,6 HLA match in RIC,8 CD34+-cell dose,13,15 CD34+-cell viability,13,16 granulocyte-macrophage colony forming unit,2,13 natural killer cells cells,6 prefreeze TNC,15 and post-thaw TNC viability.17 However, no single variable has been consistently shown to predict unit dominance across different studies, and at present, it is not therefore possible to identify which CBU will prevail before transplantation. Our data, alongside that of Ballen10 and Haspel,11 suggest that it would be reasonable to infuse the better of the 2 units first, particularly where there will be a delay between infusions.
The role of immune-mediated mechanisms in determining CBU dominance is supported by both animal and clinical data,12,14,18,19 which suggest that immune-mediated HLA mismatched responses to the recipient and nondominant CBU contribute to the emergence of single CBU dominance, which cannot reliably be predicted by the characteristics of the CBUs before transplant. Thus, understanding chimerism kinetics after DUCBT becomes increasingly important because early identification of the CBU, which will become dominant, could facilitate novel approaches to immune modulation after DUCBT, such as infusion of T-regulatory cells derived from the dominant CBU.20 Importantly, our findings suggest that the prevailing CBU can be identified by day 7 in the T-cell and B-cell lineages and by day 14 in PBMCs and granulocytes.
Although complete donor chimerism is usually achieved by single CBU dominance, we identified 3 patients in whom both CBUs persisted, with eradication of host. Stable mixed donor-donor chimerism is rare, affecting around 4% DUCBTs at 1 year after transplant, is often skewed with 1 CBU contributing more than the other, and appears to be more common after MAC.21 Mixed donor-donor chimerism may persist long term but may eventually revert to single CBU dominance, in 1 case as late as 2 years after transplant.22 The 2 CBUs involved in this mixed chimera may be phenotypically and functionally different, with the more dominant unit functioning like a single unit with the smaller having a more naive phenotype.23 Tolerance between the 2 CBUs may be more common where there is close interunit HLA matching,6,13,24 but this does not appear to be essential. Although Lamers et al25 demonstrated that T cells derived from dominant CBU were alloreactive against HLA class II alleles of the recipient leukemic cells, suggesting that graft-versus-graft alloreactivity might promote a graft-versus-leukemia effect and reduce the risk of relapse, to date, there is no clear evidence that patients with stable mixed donor-donor chimerism and intercord tolerance are at an increased risk of relapse.
Five patients in our study had persistent donor-recipient mixed chimerism, one of whom relapsed. In the context of conventional sibling and unrelated donor hemopoeitic stem cell transplantation, the presence of mixed recipient-donor chimerism is associated with an increased risk of GF26-28 and relapse28,29 and is more common after RIC. There are limited and conflicting data regarding the risk of relapse in patients with stable mixed donor-recipient chimerism after CBT. In a Japanese study of single-unit CBT after myeloablative conditioning, mixed recipient-donor chimerism within the first 90 days after transplant was not associated with an increased risk of relapse on multivariate analysis.30 Peterlin et al9 reported a higher risk of relapse but not GVHD in those not achieving complete donor chimerism after RIC DUCBT. Adults treated in the Nagoya Blood and Marrow Transplantation Group Study were found to have a higher risk of relapse in patients with mixed donor-recipient chimerism at day 56 after a single CBT.31 It would be possible to introduce early immunomodulatory interventions in recipients with persistent donor-recipient mixed chimerism after CBT, as would be considered in sibling and unrelated donor BM or PBSC transplants, such as early withdrawal of immune suppression or infusion of cell fractions (eg, CD4+ T cells32 ) expanded from the residue from the dominant unit. However, at the current time, no clear recommendations can be made given the lack of consistent evidence regarding risk of relapse in this context. Indeed, 1 patient is reported to be alive in remission with stable donor-recipient chimerism 15 years after single CBT.33
If the presence of persistent donor-recipient chimerism does not consistently identify those at risk of relapse, we considered whether falling donor chimerism could be used in identifying imminent relapse. Our data show that once complete donor chimerism was achieved after DUCBT, it was usually maintained for the duration of the study, in keeping with the Minnesota experience.5 This was not the case for 7 patients in whom recipient chimerism subsequently became detectable, and 6 of these patients relapsed. However, of the 21 patients who relapsed overall, 15 had stable chimerism at the time of relapse. These data suggest that lineage-specific chimerism monitored at the frequency used in our study is not informative in predicting relapse. It is likely that the utility of using lineage-specific chimerism to predict relapse is dependent on (1) whether the disease for which the patient was transplanted is primarily located in the bone marrow or not, (2) the speed with which relapse occurs, (3) how frequently chimerism is assessed, and (4) how rapidly immune suppression can be withdrawn. We speculate that disease specific minimal residual disease monitoring will be more useful in identifying at-risk patients who might benefit from posttransplant immunomodulation or introduction of posttransplant disease-modifying drugs.
Primary GF is a recognized limitation of CBT. Although engraftment is associated with HLA match and cell dose of infused units,34 GF cannot be reliably predicted. Early identification of those who will not engraft using chimerism would be useful clinically and enable rescue strategies (such as withdrawal of immune suppression, autologous rescue, or rescue transplant from another donor) to be implemented promptly, thereby reducing TRM. Three studies have shown that early chimerism can predict GF in patients undergoing single CBT after MAC. Moscardó et al35 found that GF was predicted by a threshold donor chimerism of 65% at day 14 in 71 adult recipients (67% GF if lower vs 3% if higher than this threshold). In 94 pediatric recipients, Elkaim et al36 reported that failure to achieve >99% donor chimerism within the first month of CBT was associated with an increased risk of nonengraftment. Chan et al37 found that 7 of 110 pediatric recipients had less than 5% donor chimerism in the third or fourth week after UCBT and all had GF.37 Previous chimerism studies in DUCBT have focused on the kinetics of CBU dominance and have excluded patients with GF (Table 4). Five patients in our study had primary GF, and chimerism data were available for 4. The chimerism kinetics in these patients was clearly different to engrafting patients, although the small sample size precludes testing for statistical significance. However, no patient with primary GF had a combined CBU chimerism in PBMC or T cells of >10% from day 21 onward, and no engrafting patients had <10% combined donor chimerism at these time points. A donor contribution to granulopoiesis was not detectable from day 21 onward in those with GF and was always present in engrafting patients. Although these data must be considered preliminary, it would be reasonable for clinicians to begin planning rescue procedures in patients with a combined donor chimerism in PBMC or T cells of less than 10% and an absence of donor derived granulopoiesis at day 21 after RIC DUCBT. Should engraftment subsequently be observed, the rescue procedure can be abandoned but if GF is confirmed, further intervention may be achieved more rapidly with the potential to reduce TRM.
This prospective phase 2 trial of CBT after RIC provides novel insights into the kinetics of donor and recipient chimerism that are clinically relevant. We showed that multilineage-specific chimerism using peripheral blood is feasible across multiple transplant centers using local laboratories even when the white cell count is low early after CBT. We confirmed that the clinical outcomes of this approach are excellent in adults with high-risk malignancies and described distinct patterns of stable chimerism after DUCBT. Further exploration of the risk of relapse in patients with persistent donor-donor and donor-recipient mixed chimerism are required to understand the utility of conventional and novel38,39 immunomodulatory therapy after DUCBT. Furthermore, our data suggest that it may be possible to use lineage-specific chimerism to predict GF earlier and instigate appropriate interventions.
Acknowledgments
The authors thank John Wagner and Juliet Barker for advice and support in writing the RIC CBT clinical protocol and patient management during the trial; Claire Harrison (Guy’s and St Thomas’ NHS Foundation Trust), David Miles (Mount Vernon Cancer Centre), Paul Silcocks (University of Liverpool), and Caroline Kelly (CR UK Clinical Trials Unit, Glasgow) for providing study oversight as the Independent Data Monitoring Committee (current and former members); Paul Smith for senior oversight of the study; and the patients and their families who took part in the study and the investigators and research staff at participating centers.
The trial was sponsored by University College London and managed by the Cancer Research UK and University College London Cancer Trials Centre. The trial was funded by grants from the Sue Harris Trust and Chugai Pharma.
Authorship
Contribution: R.H. and A.L. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; R.H. was responsible for the study concept; R.H., D.M., P.V., S.M., A.L., and P.P. designed the study; R.H., N.R., K.R., E.T., J.A.S., M.C., P.V., C.C., S.M., G.C., B.S., and D.M. recruited patients and conducted the trial locally; R.H., A.L., and L.C.-H. drafted the manuscript; A.L. analyzed the trial data; and all authors were involved in revising the manuscript and approving the final version.
Conflict-of-interest disclosure: R.H. received a travel grant from Novartis. J.S. received honoraria for speaking at educational events from Sanofi, Jazz, Gilead, Janssen, and Mallinckrodt. K.R. received honoraria for speaking at educational events from Pfizer, Jazz, Mallinckrodt, and Novartis and received travel grants from Celgene and Da-ichi Sankyo. M.C. received honoraria for speaking at educational events from Mallinckrodt. The hematology team at the CR UK and UCL CTC has received funding (which in part pays staff salary) to the sponsor and coordinate clinical trials. Funding has been awarded/used in the last 2 years from Millennium Pharmaceutics, Bristol Myers Squibb Pharmaceuticals, Amgen, Celgene, Merck Sharp and Dohme, Janssen-Cilag, and Pfizer. All remaining authors declare no competing financial interests.
Correspondence: Rachael Hough, Department of Haematology, University College Hospital, London NW1 2PG, UK; e-mail: rachaelhough@nhs.net.
References
Author notes
Requests for data sharing may be submitted to Rachael Hough (rachaelhough@nhs.net).
The full-text version of this article contains a data supplement.