Key Points
Infusion of healthy donor Treg followed by daily LD IL-2 is safe and well tolerated in patients with refractory cGVHD.
Daily LD IL-2 plus Treg DLI increases diversity of the Treg repertoire with expansion and long-term persistence of infused Treg clonotypes.
Abstract
Chronic graft-versus-host disease (cGVHD) remains a frequent cause of nonrelapse morbidity and mortality after allogeneic hematopoietic stem cell transplantation. Despite recent advances, options for steroid-refractory (SR) cGVHD are limited. In previous trials of low-dose interleukin-2 (LD IL-2), the immunomodulatory properties of regulatory T cells (Tregs) have been harnessed to treat SR-cGVHD safely and effectively. In the present study, we combined a single infusion of Treg-enriched lymphocytes (Treg DLI) from the original stem cell donor with in vivo Treg expansion using LD IL-2 (1 × 106 IU/m2 per day for 8 weeks) in 25 adult patients with SR-cGVHD. Treg were not expanded ex vivo. Treg DLI was initiated at 0.1 × 106 cells per kg patient and escalated to a maximum dose of 1 × 106 cells per kg. Treg DLI plus LD IL-2 was well tolerated and led to partial responses (PR) in 5 of 25 patients (20%) after 8 weeks of therapy. Ten additional patients (40%) had stable disease with minor responses not meeting PR criteria. Patients at all dose levels had similar Treg expansion without significant changes in CD4+ conventional T cells or CD8+ T cells. High-throughput sequencing of the T-cell receptor β locus showed selective improvement of Treg diversity. A subset of DLI-derived Treg clones showed preferential expansion at week 8 and long-term persistence 1-year postinfusion. We demonstrate for the first time that infusion of polyclonal healthy donor Tregs followed by expansion with LD IL-2 is safe in patients with SR-cGVHD, thus establishing a foundation for future adoptive Treg therapies in the posttransplant setting. This trial was registered at www.clinicaltrials.gov as #NCT01937468.
Introduction
Patients with active chronic graft-versus-host disease (cGVHD) have impaired reconstitution of CD4+CD25+CD127−FOXP3+ regulatory T cells (Tregs), which normally comprise 5% to 10% of the circulating CD4+ T-cell population and function to control inappropriate auto- and alloreactive immune responses.1-5 Preferential augmentation of Treg may be helpful in the control of cGVHD. Previously, we have used an in vivo Treg expansion approach with low-dose interleukin-2 (LD IL-2) treatment, which promotes thymic differentiation and peripheral proliferation, survival, and function of Treg.6-8 Multiple clinical trials have established that daily subcutaneous LD IL-2 administered at a dose of 1 × 106 IU/m2 per day is safe and well tolerated for prolonged periods. Importantly, this regimen induced preferential Treg expansion with objective clinical response in 50% to 60% of adults and 80% of children with steroid-refractory cGVHD (SR-cGVHD).9-12
Adoptive transfer of healthy donor Treg, with or without ex vivo expansion, has been investigated for the prevention of acute GVHD.13-16 Patients who received ex vivo–expanded third party umbilical cord blood–derived Treg in the context of a double-umbilical cord blood transplant had a lower incidence of grades 3 to 4 acute GVHD without an increase in opportunistic infections or malignant relapse when compared with contemporaneous controls undergoing double-umbilical cord blood transplant with the same conditioning and GVHD prophylaxis regimen. However, the use of adoptive Treg therapy for the treatment of cGVHD is limited to a few small case series.17,18 Three patients in one of the series also received LD IL-2 following infusion of ex vivo–expanded Treg.18 These case reports demonstrate the feasibility of this approach, but larger clinical trials are needed to evaluate the safety and efficacy of adoptive Treg therapy in reversing active cGVHD symptoms.
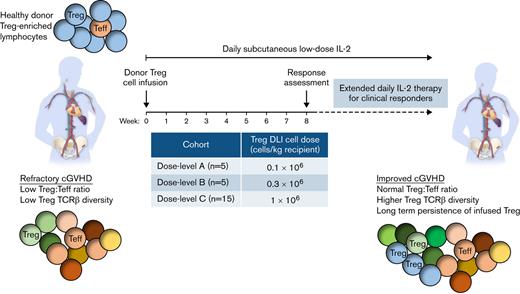
Although infusions of Treg have been safe, few studies have demonstrated persistence or expansion of adoptively transferred cells. This likely reflects the lack of a supportive environment needed to promote persistence and expansion of Treg in vivo. We hypothesized that adoptive transfer of healthy donor-derived Treg given together with daily LD IL-2 could enhance in vivo Treg expansion and undertook a phase 1 study of donor Treg infusion followed by 8 weeks of daily LD IL-2 therapy. Apheresis products obtained from the original hematopoietic stem cell donors for each patient were enriched for Treg in a 2-step process starting with CD8+/CD19+ depletion followed by CD25+ positive selection. The Treg-enriched products were infused at a dose level of 0.1 × 106 cells per kg in the first cohort and escalated to 0.3 × 106 cells per kg and 1 × 106 cells per kg in subsequent cohorts. All patients received subcutaneous LD IL-2 at a dose of 1 × 106 IU/m2 per day for 8 weeks, starting on the same day as the Treg cell infusion. Here, we report clinical and immunologic responses to this combined approach of adoptive Treg therapy with LD IL-2 in 25 adult patients with SR-cGVHD.
Methods
Phase 1 clinical protocol
This was a single-arm, single-center, phase 1 trial to assess the safety and maximum tolerated dose of Treg-enriched cells plus 8-week LD daily IL-2 in patients with SR-cGVHD. Eligibility criteria included: age ≥18 years; active cGVHD despite ≥0.25 mg/kg per day prednisone for ≥4 weeks; stable prednisone dose without addition or discontinuation of other immunosuppressive medications for ≥4 weeks prior; Eastern Cooperative Oncology Group performance status 0 to 2; and adequate renal, hepatic, pulmonary, and cardiac function. Hepatic or pulmonary dysfunction due to cGVHD was eligible. Of note, patients who did not have clinical responses to LD IL-2 on previous trials were permitted on this study (n = 5). Exclusion criteria included: prednisone requirement >1 mg/kg per day, concurrent calcineurin-inhibitor plus sirolimus, history of thrombotic microangiopathy, T-cell–targeted therapy or donor lymphocyte infusion within the 100 days prior, and active malignancy or uncontrolled infection.
Treg-enriched donor cells were administered as a single infusion starting at dose level A (0.1 × 106 viable cells per kg actual body weight recipient). Dose escalations in subsequent cohorts were targeted to 0.3 × 106 cells per kg (dose level B) and 1 × 106 cells per kg (dose level C). A cohort of 5 evaluable patients who received product meeting the release criteria entered at each dose level. Dose escalation took place when ≤1 dose-limiting toxicities were observed in a cohort of 5 patients. On the day of Treg cell infusion, daily subcutaneous IL-2 (aldesleukin, Proleukin) was initiated at 1 × 106 IU/m2 for 8 weeks. Patients with clinical benefit at the week 8 assessment could continue daily IL-2 indefinitely (Figure 1A). Dose modification of corticosteroids and other immunosuppressive medications was permitted after week 8. Patients were evaluable for toxicity at any time during the 8-week treatment period and for response after ≥4 weeks of IL-2. The trial was approved by the Dana-Farber/Harvard Cancer Center Institutional Review Board. Prior written informed consent was obtained per the Declaration of Helsinki.
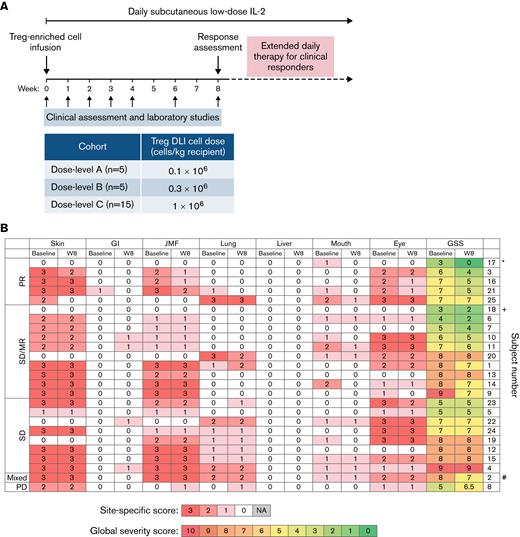
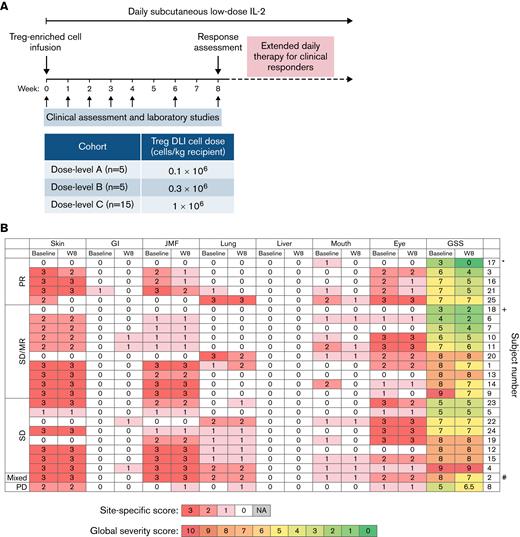
Study design and clinical response. (A) Study design. (B) Organ-specific cGVHD scores at baseline and week 8 (W8). ∗Patient 17 also had liver involvement that met PR criteria by 50% decrease in the ALT value (97 U/L at baseline, 48 U/L at week 8). +Patient 18 had polyserositis as the sole manifestation of cGVHD. #Patient 2 had lung progression based on FEV1 59% predicted at baseline and FEV1 43% at week 8 (decrease by 10% predicted absolute value of %FEV1). ALT, alanine aminotransferase; FEV, forced expiratory volume; GI, gastrointestinal; GSS, global severity score; JMF, joint/muscle/fascia; PR, partial response; SD, stable disease; SD/MR, stable disease with minor response; PD, progressive disease; NA, not applicable.
Study design and clinical response. (A) Study design. (B) Organ-specific cGVHD scores at baseline and week 8 (W8). ∗Patient 17 also had liver involvement that met PR criteria by 50% decrease in the ALT value (97 U/L at baseline, 48 U/L at week 8). +Patient 18 had polyserositis as the sole manifestation of cGVHD. #Patient 2 had lung progression based on FEV1 59% predicted at baseline and FEV1 43% at week 8 (decrease by 10% predicted absolute value of %FEV1). ALT, alanine aminotransferase; FEV, forced expiratory volume; GI, gastrointestinal; GSS, global severity score; JMF, joint/muscle/fascia; PR, partial response; SD, stable disease; SD/MR, stable disease with minor response; PD, progressive disease; NA, not applicable.
Donor Treg enrichment
Related and unrelated leukapheresis products obtained from the original hematopoietic stem cell donors for each patient underwent sequential 2-step Treg-cell enrichment using the CliniMACS instrument according to the manufacturer’s instructions (Miltenyi Biotec): (1) CD8+/CD19+ codepletion followed by (2) CD25+ positive selection. All products met lot release criteria that included ≥70% cell viability by trypan blue, negative gram stain/endotoxin, ≥70% CD4+CD25+, and ≥50% CD4+CD25+CD127− cells in the product.
Clinical assessments
cGVHD assessments using 2014 National Institutes of Health consensus criteria19 were performed at baseline, after 8 weeks of IL-2 therapy, and every 16 weeks during extended-duration therapy until 1 year from the start of IL-2 treatment or when IL-2 was stopped. Laboratory testing and flow cytometry was performed at baseline and on weeks 1, 2, 3, 4, 5, 6, and 8 and every 8 weeks while receiving extended-duration IL-2. Patient reported outcomes (PROs) were assessed using the Lee cGVHD Symptom Score.20
Flow cytometry analysis
Phenotypic analyses of lymphocytes subsets were performed at baseline (1, 2, 3, 4, 6, and 8 weeks after starting IL-2 therapy) and every 8 weeks while receiving extended-duration IL-2 therapy. Briefly, 50 μL of EDTA whole blood was subjected to red blood cell lysis using 1x BD PharmLyse (BD Biosciences) and subsequently incubated with fluorochrome-conjugated monoclonal antibodies (mAbs) as described previously.10 For intracellular marker analysis, purified peripheral blood mononuclear cells were first incubated with fluorochrome-conjugated mAbs against cell surface antigens, then fixed, permeabilized, and stained with fluorochrome-conjugated mAbs directed against intracellular antigens as described in Alho et al.5 CD4Treg were defined as CD3+CD4+CD25med-highCD127low or CD3+CD4+Foxp3+, CD4 conventional T cells (CD4Tcon) as CD3+CD4+CD25neg-lowCD127med-high; and natural killer (NK) cells as CD56+CD3−. Within CD4 T cells, coexpression of CD45RA and CD31 identified recent thymic emigrants.21,22 Proliferation was measured by expression of Ki67.23 Up to 1 million labeled cells per sample were acquired in a FACSCanto II flow cytometer (BDBiosciences) and analyzed using BD FACSDiva (BD Biosciences) and FlowJo software (Tree Star). Examples of gating of CD4Treg are shown in supplemental Figure 1. Gating and analysis strategies for simultaneous assessment of T cell subpopulations have been described previously.5,10
TCRβ repertoire analysis
Cryopreserved peripheral blood mononuclear cells from selected patients were sorted into CD4+ Treg, CD4+ Tcon, and CD8+ T cells at baseline and at multiple posttreatment time points. Additionally, a cryopreserved aliquot of the Treg-enriched donor lymphocyte infusion (Treg DLI) product for each patient was sorted into CD4+ Treg and CD4+ Tcon populations. Genomic DNA was extracted (Qiagen) from the purified populations. Survey-level T-cell receptor β (TCRβ) sequencing was performed using the ImmunoSEQ platform with subsequent diversity and clone tracking analyses using algorithms developed by Adaptive Biotechnologies.24 Details regarding the number of sorted cells, input DNA for sequencing, number of productive rearrangements, and frequencies of top 1000 clones are given in supplemental Table 1.
Statistical analysis
Patient baseline characteristics and immunophenotype data were analyzed descriptively. Overall survival (OS) was defined as the time from study entry to death from any cause. Progression-free survival was defined as the time from study entry to disease relapse, progression, or death from any cause, whichever occurred first. Patients who were alive without disease relapse or progression were censored at the time last seen alive and relapse or progression-free. The Kaplan-Meier method was used to estimate progression-free survival and OS. PRO,20 immunophenotype data, and TCR data were compared using the Wilcoxon rank-sum test for unpaired group comparison and the Wilcoxon signed-rank test for paired comparison. TCR data from a previously published phase 2 study of LD IL-2 in patients with SR-cGVHD10 was used as a control cohort for the TCR data analysis. All tests were 2-sided at the significance level of .05, and multiple comparisons were not considered. Statistical analysis was performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC) and R version 3.3.2 (the CRAN project).
Results
Patient characteristics
Twenty-five patients with a median age of 52 years (range, 23-68 years) were enrolled between January 2014 and August 2017 (Table 1): 5 were treated at 0.1 × 106 Treg-enriched cells per kg (dose level A), 5 were treated at 0.3 × 106 cells per kg (dose level B), and 5 were treated at 1 × 106 cells per kg (dose level C). As no dose-limiting toxicities were observed, dose level C was expanded to enroll 10 additional patients to ensure safety. Median time from cGVHD onset to study entry was 26.6 months (range, 3.6-83.9). At enrollment, patients had a median of 3 (range, 1-6) sites of cGVHD involvement with a median of 4 lines of prior therapies (range, 2-9). Five patients had prior IL-2 therapy.
Donor Treg product characteristics and feasibility
All donors underwent a single apheresis, and all 25 products met release criteria. Five products were collected locally at our center from matched related donors (MRD) and 20 were obtained from unrelated donors (URD) at National Marrow Donor Program collection centers and shipped to our center for processing. The number of viable total nucleated cells (TNC) obtained from MRD and URD products was similar with median viable TNC counts of 2.83 × 1010 vs 2.20 × 1010, respectively (Table 2; supplemental Table 2). Following CD8+ and CD20+ cell depletion and CD25+ cell selection, the products contained a median of 88.4% (range, 66.8-98.5) CD4+CD25+CD127lo Tregs, which represents a median 26.5-fold enrichment (Table 2; supplemental Table 2). However, the column enrichment process recovered only a median 14.9% (range, 5.6-30.5) of the Tregs in the original products. All Treg DLI products for dose levels A and B met the target dose, but only 8 of 15 products achieved the target dose of 1 × 106 Treg DLI cells per kg in dose level C (Table 2; supplemental Table 2). The difficulty in achieving this target dose may be due to the limitations of an unstimulated single apheresis product from the donors but also in part due to high body weight of the recipients (median body weight, 80.2 kg; range, 58.1-149.5).
Safety and tolerability
All 25 patients who received the Treg-enriched cell infusion and started IL-2 were evaluable for safety. During the initial 8-week treatment period, there were no infusion-related or IL-2–related dose limiting toxicities. Despite the presence of contaminating CD4+ Tcon within the Treg DLI products, there were no cases of cytokine release syndrome or GVHD flares associated with the Treg DLI infusions at any dose level. Two subjects required a 50% dose reduction of IL-2 for grade 2 flu-like symptoms and injection site reactions. IL-2–related constitutional adverse events of flu-like symptoms, fatigue, headache, nausea, and vomiting were grade 2 (n = 5) and grade 3 (n = 2). The combination of Treg DLI with daily IL-2 did not induce cytopenias or thrombotic microangiopathy. As in previous IL-2 trials,9-12 patients developed peripheral eosinophilia with a peak median absolute count of 161 cells per μL at week 4.
Clinical response and survival outcomes
There were no complete responses at week 8. Five of 25 evaluable patients (20%) had objective partial responses (PR) by week 8. Eighteen patients (72%) had stable disease (SD), including 10 patients with minimal response (MR) not meeting PR criteria (Figure 1B). One patient had a mixed response (4%), and 1 patient (4%) had progressive disease (PD). Response sites included skin (n = 2 of 20, 10%), gastrointestinal tract (n = 1 of 1, 100%), lung (n = 2 of 11, 18%; with stable FEV1 in 8 of 9 patients with pulmonary function testing data), and joint/muscle/fascia (n = 3 of 19, 16%). No patients met formal criteria for liver involvement based on liver function tests; however, patient 17 had a history of liver involvement and had an elevated alanine aminotransferase at enrollment that decreased by 50% at week 8. Patient 18 had isolated polyserositis that improved on therapy. Three of the 5 patients who had previously not responded to IL-2 alone had PR (n = 1) or SD with MR (n = 2) at week 8.
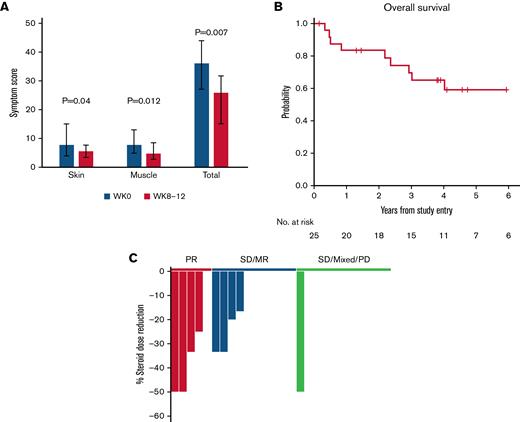
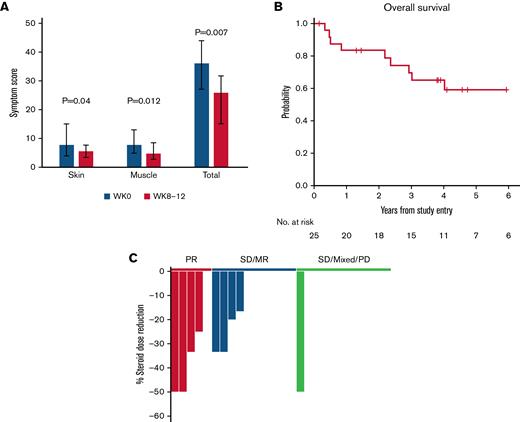
Paired pre- to posttreatment analysis was performed for each of the 8 patient reported outcome (PRO) subscale assessments. Within the entire cohort, skin and muscle symptom PROs showed the most significant improvement, and the sum of all PROs decreased from a median of 36 (range, 27-44) at baseline to 25.8 (range, 15-31.7, P = .007) at weeks 8-12 (Figure 2A).
Additional clinical outcomes. (A) Patient-reported outcomes (Lee cGVHD Symptom Scale). The skin, muscle, and total symptom scores for patients at all dose levels are shown at baseline (W0, gray) and at weeks 8 to 12 (W8-12, red). For weeks 8 to 12, the scores for weeks 8, 10, and 12 were combined and the average score was used for patients who had measurements at those timepoints. Bar graphs represent median values. Whiskers represent the interquartile range. (B) OS. (C) Steroid dose reduction by response group at week 12. Each bar represents an individual patient. The 1 patient with SD at week 8, who had 50% steroid dose reduction at week 12, achieved PR during the extension period. SD/MR, stable disease with minor response (blue); SD/MR/PD, stable disease, mixed response, progressive disease (green).
Additional clinical outcomes. (A) Patient-reported outcomes (Lee cGVHD Symptom Scale). The skin, muscle, and total symptom scores for patients at all dose levels are shown at baseline (W0, gray) and at weeks 8 to 12 (W8-12, red). For weeks 8 to 12, the scores for weeks 8, 10, and 12 were combined and the average score was used for patients who had measurements at those timepoints. Bar graphs represent median values. Whiskers represent the interquartile range. (B) OS. (C) Steroid dose reduction by response group at week 12. Each bar represents an individual patient. The 1 patient with SD at week 8, who had 50% steroid dose reduction at week 12, achieved PR during the extension period. SD/MR, stable disease with minor response (blue); SD/MR/PD, stable disease, mixed response, progressive disease (green).
Fifteen patients with clinical benefit (PR, SD/MR) elected to continue extended-duration IL-2 therapy (median 80 weeks; range, 16-226). Three additional patients achieved PR at 24, 28, and 48 weeks of therapy, respectively, leading to an overall response rate of 32%. With a median follow-up of 56 months (range, 2-87), 4-year OS was 65% (95% confidence interval: 42, 81) (Figure 2B). Nine patients died, and there were no malignant relapses. Causes of death included solid tumors (angiosarcoma and glioblastoma multiforme), infection, and progression of cGVHD (supplemental Table 3).
The median corticosteroid dose for all patients at baseline was 30 mg/day (range, 5-80 mg/day). By week 12, 4 of 5 patients with PR and 4 of 10 patients with SD/MR had significant steroid dose reduction compared with only 1 of 10 patients with SD, mixed response, or PD (Figure 2C). The 1 patient with SD later achieved PR in extended therapy. In patients continuing extended IL-2 therapy (n = 15), the median corticosteroid dose decreased to 10 mg/day (range, 0-30 mg/day; P = .0005 compared with baseline) at week 24 and to 5 mg/day (range, 0-50 mg/d) at week 56 (P = .004 compared with baseline).
Immunologic response
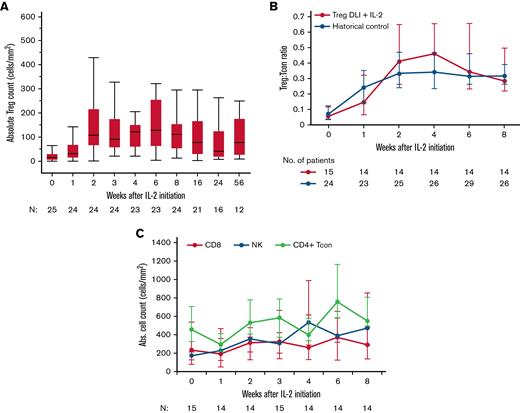
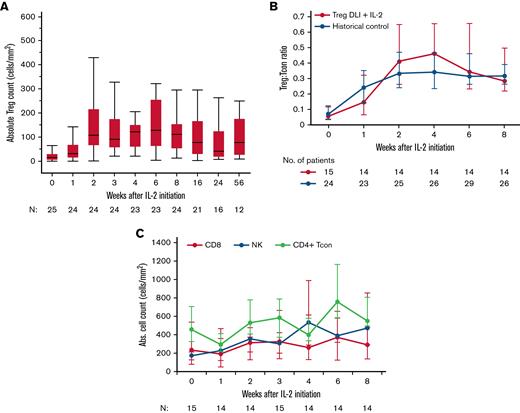
Similar to previous LD IL-2 trials, Treg DLI in combination with LD IL-2 led to a significant increase in the absolute Treg number, from a median of 15 cells per microliter (interquartile range [IQR], 6-31) at baseline to a median peak of 127 cells per microliter (IQR, 64-256) at week 6 (P < .0001 vs baseline) (Figure 3A). In patients at all dose levels combined, there was a median 10.5-fold change from baseline to week 6, and there were no significant differences in Treg expansion between the 3 Treg DLI dose levels (data not shown). Within patients at the highest Treg DLI dose level, the Treg:Tcon ratio increased from 0.07 (IQR, 0.05-0.14) at baseline to a peak of 0.47 (IQR, 0.25-0.75) at week 4 and remained elevated at 0.31 (IQR, 0.2-0.51) at week 8 (P < .0001 vs baseline). This increase was similar to that observed in our previously reported phase 2 trial of LD IL-2 alone10,25 (Figure 3B). Thus, the combination of Treg DLI at a maximum dose of 1 × 106 Treg DLI per kg with daily LD IL-2 did not result in greater in vivo Treg expansion compared with LD IL-2 alone. The NK cell number increased from 184 cells per microliter (IQR, 94-254) at baseline to a peak of 420 cells per microliter (IQR, 251-797) at week 8 (P = .0004 vs baseline). There were no significant changes in the absolute numbers of CD4+ Tcon and CD8+ T cells (Figure 3C).
Immunologic impact of LD IL-2 on lymphocytes. (A) Absolute Treg count for all dose levels combined. Box plots depict the 75th percentile, median, and 25th percentile values; whiskers represent maximum and minimum values, except for outliers (>1.5 times the interquartile range above Q3 or below Q1). (B) Treg:Tcon ratio for dose level C patients receiving Treg DLI plus LD IL-2 (red) compared with a historical control group receiving LD IL-2 alone (blue). (C) Absolute CD8+ T-cell (red), NK-cell (blue), and CD4+ Tcon (green) counts are shown for dose level C patients. Median values (dots) and the interquartile range (whisker bars) are shown at each time point. The number of patients evaluated at each time point is indicated at the bottom of each graph.
Immunologic impact of LD IL-2 on lymphocytes. (A) Absolute Treg count for all dose levels combined. Box plots depict the 75th percentile, median, and 25th percentile values; whiskers represent maximum and minimum values, except for outliers (>1.5 times the interquartile range above Q3 or below Q1). (B) Treg:Tcon ratio for dose level C patients receiving Treg DLI plus LD IL-2 (red) compared with a historical control group receiving LD IL-2 alone (blue). (C) Absolute CD8+ T-cell (red), NK-cell (blue), and CD4+ Tcon (green) counts are shown for dose level C patients. Median values (dots) and the interquartile range (whisker bars) are shown at each time point. The number of patients evaluated at each time point is indicated at the bottom of each graph.
Following Treg DLI infusion at dose level C and initiation of daily IL-2, effector memory Treg expanded first with a peak at week 3, followed by the subsequent expansion of central memory and naïve Treg (supplemental Figure 2A-B). Consistent with previous LD IL-2 trials, we observed rapid proliferation in both memory and naïve Treg subsets with a peak in Ki67 expression at week 1 (5% and 8% median increase in Ki67+ cells from baseline to week 1, respectively) (supplemental Figure 2C). The proportion of recent thymic emigrants within naïve Treg and naïve CD4+ Tcon subsets were unchanged even after 24 weeks of LD IL-2 therapy (supplemental Figure 3A-B), whereas our prior studies have shown an increase in thymic Treg output over time.12,25 This difference may be due to the fact that patients in this study had a longer median time since cGVHD onset compared with other studies (809 days vs 317 days in our phase 2 study10 and thus may have sustained more thymic injury as a consequence of prolonged cGVHD.26
TCRβ diversity
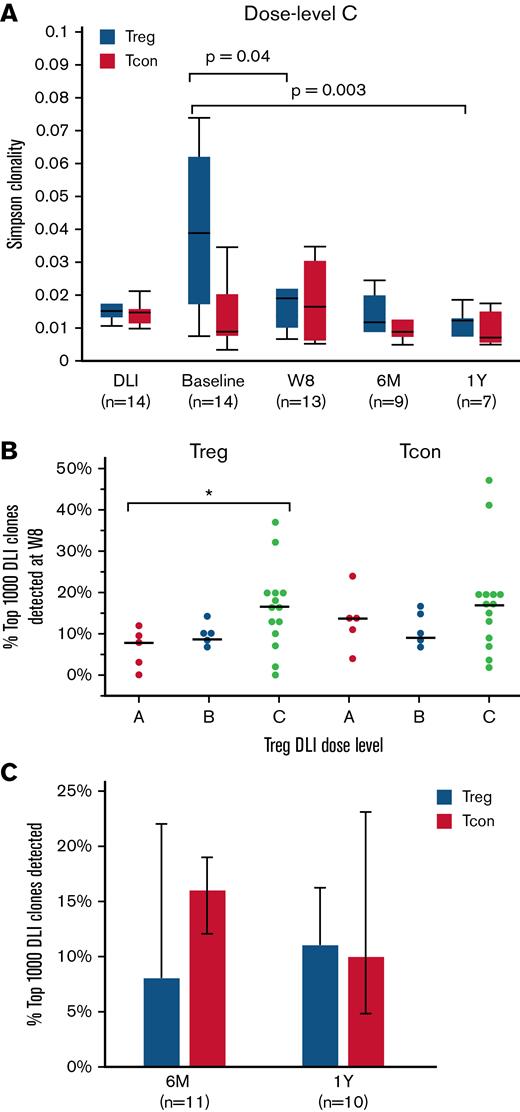
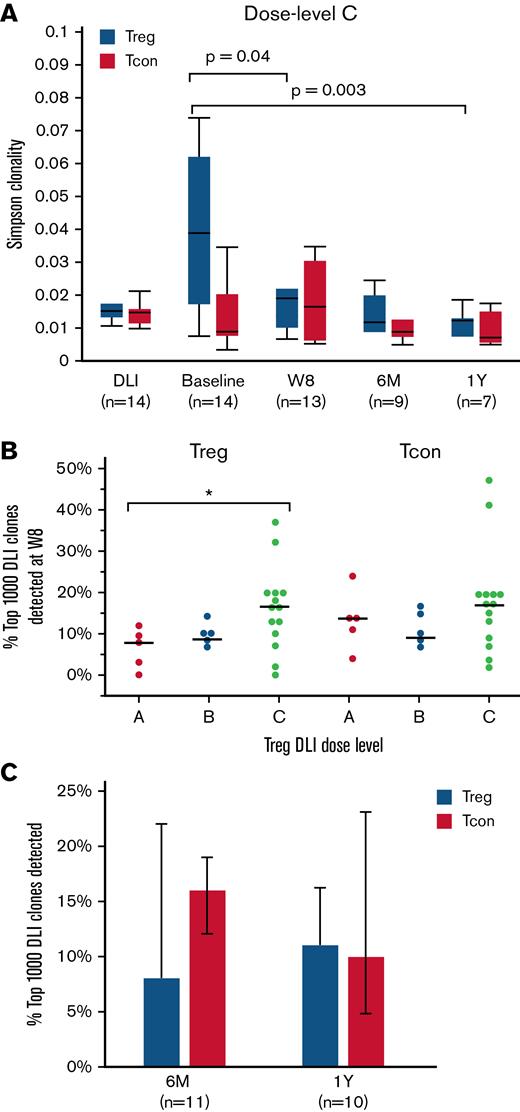
We used Simpson clonality to describe the TCRβ repertoire of sorted T-cell subsets at baseline and after Treg DLI plus LD IL-2 therapy. As a diversity metric, Simpson clonality values approaching 0 represent a more diverse and even sample, whereas values approaching 1 represent monoclonal samples. As expected, CD8+ T cells had the highest and CD4+ Tcon had the lowest Simpson clonality. Treg DLI combined with LD IL-2 led to preferential improvement of the Treg TCRβ repertoire (supplemental Figure 4). Within the Treg of dose level C patients, clonality was lowest in the healthy donor Treg DLI product (median, 0.015) and highest in patients at baseline (median, 0.039; P = .001 vs DLI). Following Treg DLI infusion and daily IL-2 therapy, the Simpson clonality within the Treg decreased to a median of 0.019 at week 8 (P = .04 vs baseline) and further improved to a median of 0.012 at both 6 months and 1 year of therapy (P = .008 and 0.003 vs baseline, respectively) (Figure 4A). A similar trend was observed with all Treg DLI dose levels combined (supplemental Figure 4). Simpson clonality within the CD4+ Tcon and CD8+ T cells did not change significantly during treatment. Regarding the improvement in Treg TCRβ diversity, it is not possible to know the contribution of the Treg DLI compared with the impact of LD IL-2 alone. However, after 1 year of therapy, patients who received Treg DLI in combination with LD IL-2 had lower Simpson clonality compared with patients who received LD IL-2 alone in a previously reported phase 2 trial10 (median, 0.013 vs 0.025; P = .005) (supplemental Figure 5). Thus, it is possible that the persistence of Treg DLI can contribute to improvement in Treg TCRβ diversity over time.
TCRβ diversity and clone tracking. (A) Simpson clonality within Treg (blue) and CD4+ Tcon (red) of dose level C patients. Diversity measurements were assessed within the Treg DLI product (DLI) and at baseline, week 8 (W8), 6 months (6M), and 1 year (1Y) after Treg DLI infusion and initiation of LD IL-2. (B) Percent of the 1000 most prevalent DLI-derived Treg and CD4+ Tcon clones that were detected at week 8 (W8) within each patient at each dose level. Dots depict individual patients. The lines represent the median value for each dose level. (C) Persistence of the top 1000 DLI-derived Treg (blue) and CD4+ Tcon (red) clones at 6 months (6M) and 1 year (1Y) after IL-2 initiation in patients receiving extended IL-2 therapy. Box plots depict the 75th percentile, median, and 25th percentile values; whiskers represent maximum and minimum values except for outliers (>1.5 times the interquartile range above Q3 or below Q1). Bar graphs represent median values; whiskers represent the interquartile range. The number of patients evaluated at each time point is indicated at the bottom of the image. Two-sided Wilcoxon signed-rank test was used to assess significance.
TCRβ diversity and clone tracking. (A) Simpson clonality within Treg (blue) and CD4+ Tcon (red) of dose level C patients. Diversity measurements were assessed within the Treg DLI product (DLI) and at baseline, week 8 (W8), 6 months (6M), and 1 year (1Y) after Treg DLI infusion and initiation of LD IL-2. (B) Percent of the 1000 most prevalent DLI-derived Treg and CD4+ Tcon clones that were detected at week 8 (W8) within each patient at each dose level. Dots depict individual patients. The lines represent the median value for each dose level. (C) Persistence of the top 1000 DLI-derived Treg (blue) and CD4+ Tcon (red) clones at 6 months (6M) and 1 year (1Y) after IL-2 initiation in patients receiving extended IL-2 therapy. Box plots depict the 75th percentile, median, and 25th percentile values; whiskers represent maximum and minimum values except for outliers (>1.5 times the interquartile range above Q3 or below Q1). Bar graphs represent median values; whiskers represent the interquartile range. The number of patients evaluated at each time point is indicated at the bottom of the image. Two-sided Wilcoxon signed-rank test was used to assess significance.
TCRβ clone tracking
Next, we examined the fate of the infused Treg and CD4+ Tcon within the Treg DLI product by clone tracking analyses. First, we asked how many of the most prevalent 1000 unique Treg clones in the Treg DLI product were still detectable after 8 weeks of LD IL-2 therapy. Patients at dose level C had the highest percentage of prevalent DLI-derived Treg clones that persisted at week 8 (median, 17% vs 8% for dose level A; P = .04). The proportion of CD4+ Tcon derived from the Treg DLI product at week 8 was not significantly different between the 3 dose levels (Figure 4B). In patients continuing extended therapy, we observed long-term persistence of DLI-derived Treg and CD4+ Tcon clones, with 7% to 15% of the most prevalent DLI-derived clones detected 6 months and 1 year after infusion (Figure 4C).
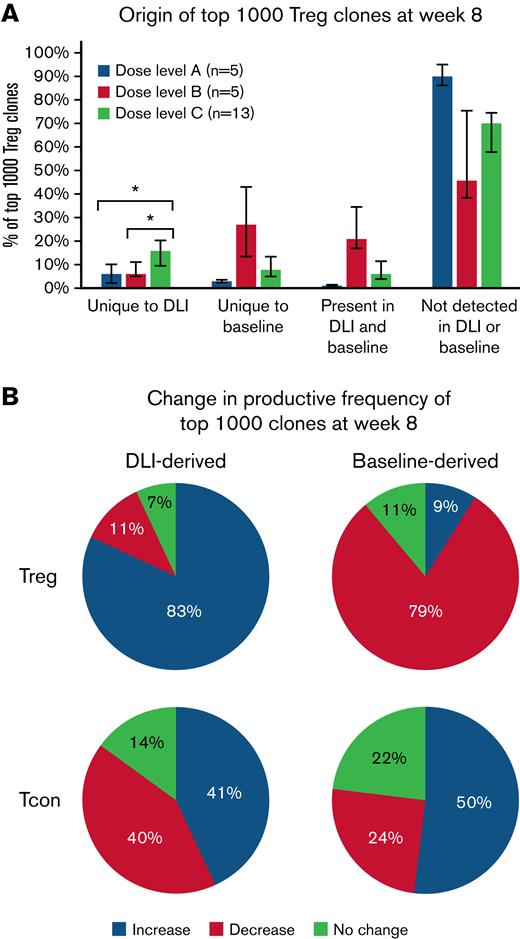
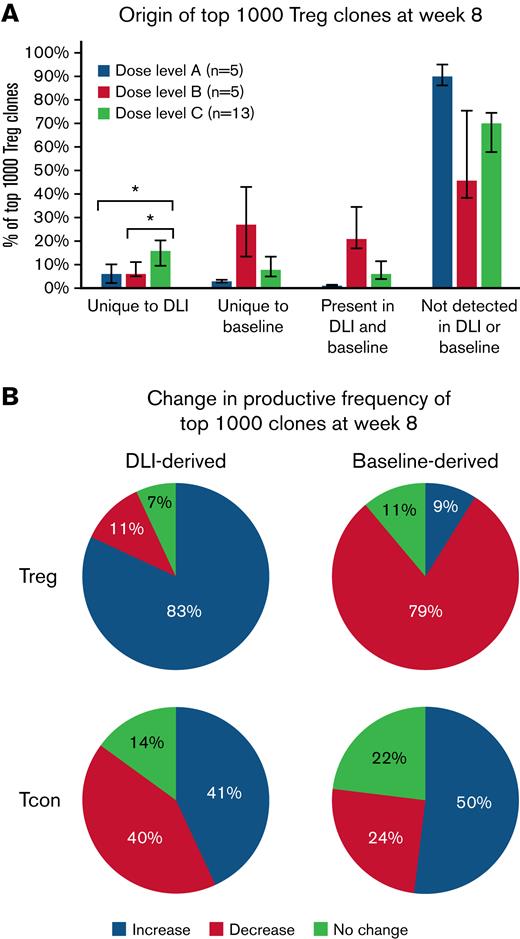
We also examined the origin of the most prevalent 1000 Treg clones at week 8 following Treg DLI infusion plus IL-2 therapy. Clones could be derived from the infused Treg DLI product, from the patient’s baseline pool of Treg clones. or could be of unknown origin. These “emergent” clones may have been present at undetectable levels in either the Treg DLI product or the patient’s baseline pool or may have arisen de novo during IL-2 therapy. As the Treg DLI was obtained from the original stem cell donor, some clones were detected in both the Treg DLI product and in the patient’s preexisting baseline population. However, even at the highest dose level of infused Treg DLI, the TCR overlap (Morisita index) between the DLI and baseline samples was low compared with the overlap between baseline and week 8 samples within each patient (supplemental Figure 6). As expected, patients in dose level C had the highest percentage of clones uniquely derived from the Treg DLI product (median, 6% vs 6% vs 17% for dose levels A, B, and C, respectively; P = .009). In all dose levels, the majority of the prevalent week 8 clones were emergent Treg clones (Figure 5A).
Origin of dominant clones within Treg and CD4+Tcon at week 8 after starting Treg DLI plus IL-2 therapy. (A) Percentage of the 1000 most prevalent Treg clones at week 8 derived from the DLI product, baseline, present in DLI and baseline, or not detected in DLI or baseline are shown for each dose level. Dose level A, blue; dose level B, orange; dose level C, gray. Bar graphs depict median values and whiskers represent the interquartile range. ∗ P < .01. (B) Behavior of the top 1000 week 8 clones derived from the Treg DLI product (DLI-derived) or the patients’ baseline pool (Baseline-derived). The median percentage of clones with increased productive frequency (expand, blue), decreased productive frequency (contract, red), or no change in productive frequency (green) is shown for patients at all dose levels combined (n = 23).
Origin of dominant clones within Treg and CD4+Tcon at week 8 after starting Treg DLI plus IL-2 therapy. (A) Percentage of the 1000 most prevalent Treg clones at week 8 derived from the DLI product, baseline, present in DLI and baseline, or not detected in DLI or baseline are shown for each dose level. Dose level A, blue; dose level B, orange; dose level C, gray. Bar graphs depict median values and whiskers represent the interquartile range. ∗ P < .01. (B) Behavior of the top 1000 week 8 clones derived from the Treg DLI product (DLI-derived) or the patients’ baseline pool (Baseline-derived). The median percentage of clones with increased productive frequency (expand, blue), decreased productive frequency (contract, red), or no change in productive frequency (green) is shown for patients at all dose levels combined (n = 23).
Next, we asked whether there were differences in the expansion or contraction of the individual prevalent clones at week 8 depending on their origin. We compared the frequency of a specific clone among all clones within a sample (productive frequency) for clones in the Treg DLI and baseline samples with week 8 samples. Interestingly, the majority of clones derived from the Treg DLI product had an increase in productive frequency at week 8 (median, 83%), whereas the productive frequency had decreased for the majority of clones derived from the baseline population (median, 79%) (Figure 5B). This observation suggests that there is a subset of Treg clones within the healthy Treg DLI products that undergo antigen-specific expansion following infusion into cGVHD patients receiving daily LD IL-2. Preferential expansion of DLI-derived clones was not seen within the most prevalent clones within the CD4+ Tcon population at week 8 (Figure 5B).
Discussion
Most agents for treating SR-cGVHD are broadly immunosuppressive and directed to inhibition of effector T cells and B cells.27-30 Based on the observation that patients with cGVHD had lower numbers of Treg, LD IL-2 was developed as a therapy for refractory cGVHD with the rationale that preferential in vivo expansion of Treg and restoration of a healthy balance of the Treg-to-Teff ratio would induce immune tolerance and ameliorate cGVHD manifestations.9 Over the last decade, we have demonstrated safety and efficacy of daily LD IL-2 in the treatment of SR-cGVHD, with overall response rates of 50% to 60% in adults and 80% in children.10-12 We asked whether infusion of healthy donor lymphocytes enriched for Treg in combination with daily LD IL-2 would enhance in vivo Treg expansion and further improve clinical responses in patients with refractory cGVHD.
In this phase 1 trial, we have demonstrated safety and tolerability of adoptive Treg DLI for the first time in patients with refractory cGVHD. The DLI products contained a median of 88% Treg and were enriched from single unstimulated apheresis collections from both related and unrelated donors. We observed similar viable TNC counts between the donor sources despite variability in collection centers and transport time for the URD products. Importantly, these Treg DLI products were infused fresh without an ex vivo expansion step. Although the presence of contaminating CD4+ Tcon were a concern, there were no infusion-related adverse events and no GVHD flares or cytokine release symptoms observed following the infusions during the initial in vivo expansion phase with LD IL-2. Moreover, TCRβ clone tracking revealed no preferential expansion of the DLI-derived CD4+ Tcon in vivo.
Regarding efficacy, only 5 of 25 evaluable patients (20%) treated on this study had objective clinical improvement after 8 weeks of therapy. This response rate is lower than in our previous trials of LD IL-2, though the response rate including the 10 patients with minor responses (some of whom achieved formal PR on extended IL-2 therapy) is similar to previously observed response rates (50% to 60%). The lower response rate may be explained by more advanced or refractory cGVHD. For example, the time from cGVHD onset to study enrollment on this study was more than twice that of patients on our prior phase 2 trial. In addition, 11 of 25 (44%) patients had pulmonary manifestation of cGVHD. However, 3 of the 5 patients previously treated with IL-2 alone had PR (n = 1) or SD with MR (n = 2) at week 8 on this study, suggesting that adoptive Treg therapy in conjunction with LD IL-2 may provide added benefit in some patients.
This limited efficacy may also be due to the constraints of using a single donor apheresis product, particularly in patients with high body weight. Without an expansion step, we did not meet the target cell dose for 7 of the 15 patients at the highest planned dose level (1 × 106 Treg DLI cells per kg). With infusion of a relatively small dose of Treg, subsequent expansion of Treg in vivo was similar to what has been achieved with LD IL-2 alone. It is possible that the maximal dose achieved in this study is insufficient to induce clinical improvement of cGVHD manifestations. In a study of ex vivo expanded umbilical cord blood Treg for acute GVHD prevention, Brunstein et al infused Treg doses ranging from 3 to 100 × 106 Treg/kg.14 Other studies using ex vivo expanded autologous Treg for the treatment of type 1 diabetes and systemic lupus erythematosus infused Treg doses ranging from 10 to 20 × 106 Treg per kg and 1 × 108 total Treg, respectively.31,32
Consistent with our previous analysis of patients receiving LD IL-2 alone,25 Treg TCRβ repertoire diversity improved significantly after Treg DLI infusion and daily LD IL-2 therapy for 8 weeks. Patients receiving extended-duration LD IL-2 had continued improvement in Treg TCR diversity. In contrast, IL-2 therapy did not change TCR diversity within the CD4+ Tcon and CD8+ T cells. Although long-term persistence of adoptively transferred Treg has been inferred from long-lived detection of isotope-labeled DNA extracted from circulating Treg at 1-year postinfusion in a different clinical setting,33,34 tracking of unique TCR sequences within the infused Treg DLI clones provides more definitive evidence of Treg expansion and persistence. By TCR clone tracking, we observed that both DLI-derived Treg and CD4+ Tcon clones could be detected in the peripheral blood at 1-year postinfusion. However, only Treg clones showed evidence of expansion in vivo, and contaminating CD4+ Tcon within the Treg DLI products did not expand, consistent with the selective effect of LD IL-2 on Tregs.
In conclusion, this is the largest reported experience of adoptive Treg transfer in patients after allogeneic hematopoietic stem cell transplantation. We have shown that infusion of healthy donor Treg followed by further in vivo expansion with LD IL-2 is safe and well tolerated in patients with refractory cGVHD. However, this approach did not improve clinical response rates compared with IL-2 alone. In future studies, incorporating higher Treg doses after ex vivo expansion of purified Treg combined with further in vivo expansion using LD IL-2 and earlier initiation of therapy following the onset of cGVHD may lead to higher clinical response rates. Importantly, we have demonstrated the safety and feasibility of donor Treg infusions in the posttransplant setting. This foundation will enable future applications of adoptive Treg therapy for tolerance induction and cGVHD prevention.
Acknowledgments
The authors thank the patients who participated in this trial and their families. The authors thank the Connell and O’Reilly Families Cell Manipulation Core Facility for manufacturing Treg DLI products and the Pasquarello Tissue Bank in Hematologic Malignancies for prospective collection and processing of serial blood samples.
This work was supported by National Institutes of Health (NIH), National Cancer Institute grants P01CA229092, P01CA142106, and P30CA006516. Prometheus Laboratories Inc. provided aldesleukin for patients enrolled on this clinical trial.
Authorship
Contribution: S.N., J.K., J.F.L., and J.R. conceived and designed the study; J.K., E.P.A., C.S.C., V.T.H., J.H.A., and R.J.S. provided patients; J.S.W., S.N., J.W., C.G.R., S.C.R., S.K., A.B., and A.C.A. collected and assembled data; H.T.K. analyzed and interpreted data and performed statistical analysis; and all authors wrote the manuscript.
Conflict-of-interest disclosure: J.K. receives research support from BMS, Miltenyi Biotec, Novartis, Clinigen, and Regeneron; serves on scientific advisory boards for Therakos, Cugene, and Biolojic Design; and consults for Amgen, Gentibio, Equillium, EMD Serono/Merck, and Moderna. J.R. receives research funding from Amgen, Equillium, Kite/Gilead, and Novartis and serves on data safety monitoring committees for AvroBio and scientific advisory boards for Akron Biotech, Clade Therapeutics, Garuda Therapeutics, Immunitas Therapeutics, LifeVault Bio, Novartis, Rheos Medicines, Talaris Therapeutics, and TScan Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: John Koreth, D2029 Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: john_koreth@dfci.harvard.edu.
References
Author notes
For original data, please contact john_koreth@dfci.harvard.edu.
The full-text version of this article contains a data supplement.
J.S.W. and S.N. are joint first authors.