TO THE EDITOR:
Approximately 70% of cases of acute myeloid leukemia (AML) are diagnosed in adults ≥60 years of age.1 There is substantial variation in the health status of older adults with AML and their preferences for treatment outcomes, which makes treatment selection challenging.2 Shared decision making (SDM) is a key component of patient-centered care.3 To achieve SDM, patients must understand the nature of their disease (including the prognosis) and its treatment options (including the risks and benefits).4 In addition, patient values must be incorporated into treatment decisions.4 The rapid onset and progression of AML can lead to decisional time constraints for health care professionals and overwhelming emotional challenges for patients, which presents additional barriers to SDM.5,6
We previously found that many older patients believed they were not well informed about their disease and its treatment options.7 Older patients and their oncologists identified aging-related vulnerabilities in physical function and cognition as important in influencing their decision making, although these factors were not systematically or formally assessed.7 More than half of patients with hematologic malignancies overestimated their prognosis compared with their oncologists’ estimates.8 In response to these gaps, we developed a patient-centered communication tool (University of Rochester-Geriatric Oncology Assessment for Acute Myeloid Leukemia [UR-GOAL]), which addresses aging-related vulnerabilities, patient values, and prognostic awareness among older patients with AML. The purpose of this single-arm pilot study was to adapt the UR-GOAL communication tool, with an ultimate goal of improving SDM between older adults with AML and their oncologists.
We recruited older patients with AML from an academic cancer center. Eligible patients were (1) ≥60 years of age; (2) had been diagnosed with AML in the past year; (3) were English-speaking; and (4) had been able to provide informed consent. Although this tool was specifically designed for patients with newly diagnosed AML, we wanted to seek feedback from patients with an established AML diagnosis first, given that patients with newly diagnosed AML are often overwhelmed and in distress. The University of Rochester Research Subjects Review Board approved the study. We followed the Consolidated Criteria for Reporting Qualitative Research (COREQ) checklist when reporting our study (supplemental Table 1).9
After obtaining informed consent, we collected demographic and clinical information. Patients completed the UR-GOAL communication tool. Then, a 30- to 60-minute semistructured interview was conducted with each patient, either virtually or in a private space in the hospital (supplemental Figure 1). Audio recordings of all interviews were transcribed.
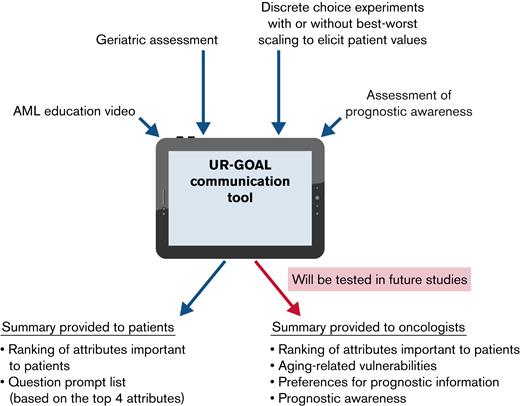
The UR-GOAL communication tool consists of 4 components (Figure 1): (1) a 5-minute AML animated educational video; (2) a geriatric assessment to evaluate aging-related vulnerabilities (self-reported and objective measures to assess physical function, nutritional status, social support, cognition, and number of medications)10; (3) preference elicitation techniques to reveal patient values (choice-based conjoint analysis [CBC] and best-worst scaling [BWS]; supplemental Figure 2; supplemental Table 2); and (4) prognostic awareness and preference questions on prognostic information.8,11,12 A summary report that contains geriatric assessment and patient values and a list of question prompts are generated for patients. In this initial phase of adaptation, the summary was focused on patients, and therefore oncologists did not receive the summary report.
We used descriptive statistics to summarize demographic, cancer, and clinical characteristics. We analyzed the interviews using deductive thematic analyses.13 Two independent coders (A.-M.C. and K.P.L.) analyzed all transcripts using the MAXQDA 2018 software (VERBI Software GmbH, Berlin, Germany).
We screened 23 patients, 5 of whom were not approached (4 had declining/poor heath, and 1 was overwhelmed by cancer care). Of the 18 patients approached, 15 provided consent, yielding a recruitment rate of 83.3%. Two patients were not interested, and 1 patient was overwhelmed by cancer care. All patients who consented to participate completed the study (retention rate of 100%).
The mean age of the 15 patients was 69.1 years (standard deviation, 5.6; range, 62-80) and median time from AML diagnosis to interview was 192 days (quartile 1 [Q1]-Q3: 80-213). Other demographics and clinical characteristics are shown in Table 1. For each of the following 3 content areas (AML education video, communication tool, and summary report), we identified up to 3 themes (supplemental Table 3).
Most patients (11 of 15) had positive reactions to the informational video. They found it to be informative, succinct, and comprehensible. Participants commented that the video was a good first exposure to the science of AML for someone who does not know where to begin.
Although the video’s content received majority positive feedback, the patients had mixed preferences about when the video should be viewed and the appropriateness of video as a mechanism for education about the disease. Inclusion of prognostic information was helpful, but 3 patients thought the information was too sensitive to be communicated through video.
More than half of the participants (5 of 8) reported confusion pertaining to the online survey interface for the CBC. The BWS technique was described by participants as “friendlier” and “clearer.” Among the participants who completed both CBC and BWS (n = 7), 5 preferred the BWS.
The communication tool prompted self-reflection among the participants. Participants mentioned the tool gave them “lots to think about.” Most of what patients disclosed revolved around their prognosis and their thought processes when considering treatment factors.
Three patients who completed the CBC felt that the ranking of attributes in their summary report was inaccurate. It was suggested adding an open-ended comment box at the end of the tool to enable patients to insert clarifications before the report was provided to their clinician.
Definitions of the attributes listed in the report were unclear to some patients. Patients suggested that descriptions of each attribute should be added to make results more understandable.
Patients (9 of 15) enjoyed and valued the report’s tailored list of questions to ask their oncologists. Patients thought it provided a useful guide for facilitating conversations regarding treatment and felt validated in knowing that they were not the only ones with these questions.
The UR-GOAL communication tool provides education followed by assessment of aging-related vulnerabilities, patient values, and prognostic awareness. Most patients found the educational video to be succinct and informative. Patients also liked the question prompts in the summary report, which can equip them with questions for discussion with their oncologists based on their preferences. BWS and CBC allow for self-reflection; BWS was preferred over CBC because of the ease of use.
In prior studies of AML decision tools, aids, and programs, the interventions tested included trained facilitators who provide support in making treatment decisions, a web-based tool and nurse-delivered telephone support, actual patient videos sharing experiences and reflections, and AML educational videos.14-17 None of the tools collected information about a patient’s aging-related vulnerabilies, personal values, and prognostic awareness. When used in treatment decision making for patients with newly diagnosed prostate cancer, a CBC decision aid was found to increase patients’ satisfaction and reduce decisional regret in a multicenter study.18 In the context of AML, BWS has been used to elicit the concerns of patients and caregivers.19 To the authors’ knowledge, no CBC- or BWS-based tools have been developed to specifically address the needs of older patients with AML and assist with making treatment decisions in real-time. In response to patient feedback, several changes were made to the communication tool to be used for future studies (supplemental Table 4).
Our study has several strengths. First, we included a vulnerable and underrepresented population (ie, older adults with AML), and their feedback enabled us to adapt a tool that is patient centered. Second, our study procedures can be conducted in person or virtually, providing flexibility and comfort amid the COVID pandemic. Our study also has limitations. All participants were recruited from an academic cancer center. Survivorship bias may be present; those with poor or declining health were not approached. All patients were non-Hispanic White and had received AML treatments. Our findings may not apply to marginalized populations or to those who do not receive any AML-directed treatment. Finally, ∼65% of older patients screened were enrolled, reflecting the vulnerabilities of this population as well as the potential for rapid decline caused by AML and its treatment. These factors, however, should not deter participation of this population in clinical trials but should be carefully considered by researchers in designing studies.
In summary, patients’ feedback from this study has informed the adaptation of a novel patient-centered communication tool for older patients with AML and their oncologists. Our ongoing studies will evaluate whether the UR-GOAL is feasible for use in clinical practice (registered on http//:clinicaltrials.gov as #NCT04625413) and improve SDM and communication (#NCT05335369).
Acknowledgments: The authors thank Susan Rosenthal, MD for editorial assistance.
This work was supported by National Institutes of Health (NIH); National Cancer Institute grants UG1CA189961, K99CA237744l, and R00CA237744 (K.P.L.); NIH, National Institute of Aging grants R33AG059206 (H.D.K., W.D.) and K76AG064431 (M.L.W.); and a Wilmot Research Fellowship Award (K.P.L.). The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Contribution: E.W. collected, analyzed, and interpreted the data and wrote and approved the submitted manuscript; C.S. collected, analyzed, and interpreted the data and wrote the manuscript; A.-M.C. analyzed and interpreted the data and wrote and approved the submitted manuscript; H.D.K., M.W., and S.N., conceived and designed the study and reviewed and approved the submitted manuscript; D.R.R., W.D., A.M., J.H.M., J.L., E.H., K.O’D., T.W.L., A.E.-J., and M.L.W. interpretated the data and reviewed and approved the submitted manuscript; K.P.L. conceived and designed the study and collected, analyzed, and interpreted the data and wrote and approved the submitted manuscript.
Conflict-of-interest disclosure: K.P.L. has served as a consultant to Pfizer and Seattle Genetics and has received honoraria from Pfizer. T.W.L. has received personal fees for consulting or membership on advisory boards from AbbVie, Agios, AstraZeneca, Amgen, Astellas, CareVive, BMS/Celgene, Daiichi-Sankyo, Heron, Flatiron, Pfizer, and Seattle Genetics; royalties from UpToDate; speakers bureau fees from Agios, AbbVie, and BMS/Celgene; and grants and/or research contracts from the American Cancer Society, AstraZeneca, BMS, Jazz Pharmaceuticals, the National Institution of Nursing research (NINR), NIH, and Seattle Genetics. M.L.W. reports conflicts of interest outside of the submitted work (royalties from UpToDate and immediate family member is an employee of Genentech with stock ownership). The remaining authors declare no competing financial interests.
Correspondence: Kah Poh Loh, Division of Hematology/Oncology, Department of Medicine, James P. Wilmot Cancer Institute, University of Rochester Medical Center, 601 Elmwood Ave, Box 704, Rochester, NY 14642; e-mail: kahpoh_loh@urmc.rochester.edu.
References
Author notes
Contact the corresponding author for data sharing (kahpoh_loh@urmc.rochester.edu).
The full-text version of this article contains a data supplement.