TO THE EDITOR:
Platelets are produced by megakaryocytes in the bone marrow. Failure to produce enough platelets or enhanced clearance results in thrombocytopenia, and a bleeding tendency if the platelet count becomes too low.
Platelets contain many N- and O-linked glycans, which are normally capped by a sialic acid group. Loss of sialic acid from surface carbohydrates is a clearance signal for senescent platelets,1 but desialylation is also frequently observed in acquired thrombocytopenia.2,3 Recently, genetic variants in the glucosamine (UDP-N-acetyl)-2-epimerase/N-acetylmannosamine kinase (GNE) gene were identified in patients with congenital macrothrombocytopenia.4-6 Two of the identified GNE variants were associated with platelet hyposialylation, but platelet sialylation was not investigated in the context of the other variants.
GNE is the rate-limiting enzyme in the endogenous synthesis of sialic acid. Mutations in GNE are associated with adult-onset myopathy. Although low platelet counts have been reported in isolated cases of GNE myopathy,7 most patients have normal platelet counts. Hence, the relationship between GNE mutations and macrothrombocytopenia is incompletely understood. Here, we present 2 cases with congenital macrothrombocytopenia, a severe bleeding tendency, and a mutation in the GNE gene. We provide evidence that the mutation results in impaired GNE activity and is associated specifically with hepatic clearance of hyposialylated platelets.
Two White European female siblings (P1 and P2) presented with spontaneous mucocutaneous bleeds and menorrhagia, which required frequent hospitalization. Their history showed a persistent severe macrothrombocytopenia from 1 week of age (Table 1), with giant platelets in a peripheral blood smear (Figure 1A). Both patients received platelet transfusions on demand to restore hemostasis. Surface expression of the GPIb-V-IX complex was reduced (Figure 1B). Analysis of platelet reactivity toward agonists indicated normal responses (Figure 1C), although P-selectin expression was slightly reduced with 2 agonists. Their nonconsanguineous parents did not have a bleeding tendency and had normal platelet counts (207 × 109/L and 341 × 109/L). Clinical quadruple whole-exome sequencing indicated a homozygous missense variant (c.1259G>A; p. R420Q) in the GNE gene. The allele frequency of this variant is 8 × 10-6 in the total population according to the gnomAD browser8 and it is predicted to be “probably damaging.”
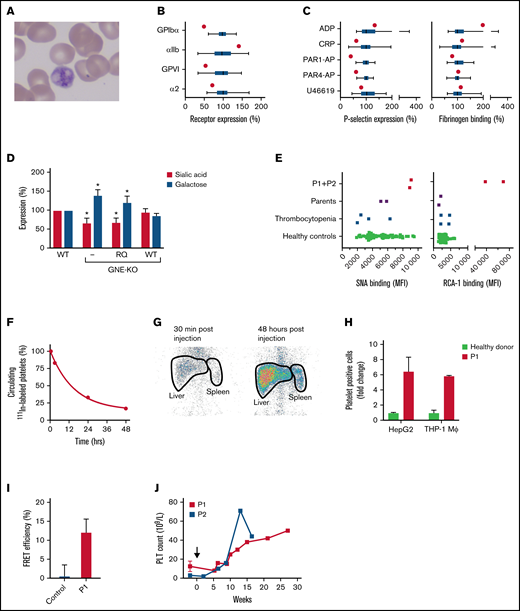
The 420Q-GNE variant is associated with platelet hyposialylation and enhanced hepatic clearance. (A) Peripheral blood smear of P1. (B) Relative expression of the glycoprotein Ib-V-IX complex (GPIbα), fibrinogen receptor αIIbβ3 (αIIb), and collagen receptors glycoprotein VI (GPVI) and α2β1 (α2) in P1 (red dots) and 49 healthy controls measured with flow cytometry. Data of healthy controls (green) are expressed in box and whiskers. Average median fluorescent intensity values in controls were set at 100%. Whiskers represent 2.5th and 97.5th percentiles. (C) Platelet reactivity measured with flow cytometry in P1 (red dot) and 49 healthy controls (green box and whiskers). Platelets were stimulated with 60 μM ADP, 25 μM protease-activated receptor (PAR)-1 activating peptide (PAR1-AP) SFLLRN, 250 μM PAR4-AP AYPGKV, 1 μg/mL cross-linked collagen-related peptide (CRP-XL), or 5 μM U-46619, fixated and analyzed for expression of P-selectin as marker for granule secretion and fibrinogen binding as marker for αIIbβ3 activation. Average median fluorescent intensity values in controls were set at 100%. Whiskers represent 2.5th and 97.5th percentiles. (D) Wild-type (WT), GNE-deficient (KO), and GNE-deficient HEK293 cells overexpressing either recombinant WT-GNE, or recombinant 420Q-GNE (RQ) were incubated with fluorescein-conjugated SNA lectin for analysis of sialic acid expression, or fluorescein-conjugated RCA-1 lectin for analysis of galactose expression. Lectin binding was assessed with flow cytometry. Data were normalized on lectin binding in WT HEK293 cells (n = 3). *P < .05, error bars represent the standard deviation. (E) Platelet sialic acid exposure was measured with fluorescein-SNA lectin and galactose exposure was measured with fluorescein-RCA-1 lectin on a flow cytometer in both P1 and P2, their parents, 4 thrombocytopenic patients, and 68 healthy controls. MFI, median fluorescent intensity. (F) Autologous platelets were labeled with 111In-tropolone and injected into P1. Platelets were collected at different time points to determine platelet half-life. The fraction 111In-labeled platelets at 30 minutes after injection was set at 100%. Data represent the relative proportion of 111In-labeled platelets at each time point. (G) Anterior static single-photon emission computed tomography scans of the abdomen of P1 were made with a Symbia T2 γ camera (Siemens, Erlangen, Germany) at indicated time points to quantify platelet sequestration. Radioactivity in liver and spleen regions (thick black lines) was assessed as the percentage of total radioactivity. Spleen:liver radioactivity ratio <0.8 indicates hepatic sequestration. (H) Celltracker Deep Red-labeled platelets of P1 (red) (n = 2) and healthy controls (green) were incubated with THP-1 macrophages (n = 4) and HepG2 hepatocytes (n = 3). Data are normalized on the number of platelet-binding cells with control platelets. (I) Platelets from P1 (red) (n = 3) or healthy controls (green) (n = 3) were labeled with fluorophore conjugated anti-GPIbα Fab′-fragments (6B4). Fluorophore lifetime in presence and absence of an acceptor was assessed and used to calculate fluorescence resonance energy transfer efficiency. (J) Change in platelet count in P1 and P2 after initiation of treatment with romiplostim (black arrow).
The 420Q-GNE variant is associated with platelet hyposialylation and enhanced hepatic clearance. (A) Peripheral blood smear of P1. (B) Relative expression of the glycoprotein Ib-V-IX complex (GPIbα), fibrinogen receptor αIIbβ3 (αIIb), and collagen receptors glycoprotein VI (GPVI) and α2β1 (α2) in P1 (red dots) and 49 healthy controls measured with flow cytometry. Data of healthy controls (green) are expressed in box and whiskers. Average median fluorescent intensity values in controls were set at 100%. Whiskers represent 2.5th and 97.5th percentiles. (C) Platelet reactivity measured with flow cytometry in P1 (red dot) and 49 healthy controls (green box and whiskers). Platelets were stimulated with 60 μM ADP, 25 μM protease-activated receptor (PAR)-1 activating peptide (PAR1-AP) SFLLRN, 250 μM PAR4-AP AYPGKV, 1 μg/mL cross-linked collagen-related peptide (CRP-XL), or 5 μM U-46619, fixated and analyzed for expression of P-selectin as marker for granule secretion and fibrinogen binding as marker for αIIbβ3 activation. Average median fluorescent intensity values in controls were set at 100%. Whiskers represent 2.5th and 97.5th percentiles. (D) Wild-type (WT), GNE-deficient (KO), and GNE-deficient HEK293 cells overexpressing either recombinant WT-GNE, or recombinant 420Q-GNE (RQ) were incubated with fluorescein-conjugated SNA lectin for analysis of sialic acid expression, or fluorescein-conjugated RCA-1 lectin for analysis of galactose expression. Lectin binding was assessed with flow cytometry. Data were normalized on lectin binding in WT HEK293 cells (n = 3). *P < .05, error bars represent the standard deviation. (E) Platelet sialic acid exposure was measured with fluorescein-SNA lectin and galactose exposure was measured with fluorescein-RCA-1 lectin on a flow cytometer in both P1 and P2, their parents, 4 thrombocytopenic patients, and 68 healthy controls. MFI, median fluorescent intensity. (F) Autologous platelets were labeled with 111In-tropolone and injected into P1. Platelets were collected at different time points to determine platelet half-life. The fraction 111In-labeled platelets at 30 minutes after injection was set at 100%. Data represent the relative proportion of 111In-labeled platelets at each time point. (G) Anterior static single-photon emission computed tomography scans of the abdomen of P1 were made with a Symbia T2 γ camera (Siemens, Erlangen, Germany) at indicated time points to quantify platelet sequestration. Radioactivity in liver and spleen regions (thick black lines) was assessed as the percentage of total radioactivity. Spleen:liver radioactivity ratio <0.8 indicates hepatic sequestration. (H) Celltracker Deep Red-labeled platelets of P1 (red) (n = 2) and healthy controls (green) were incubated with THP-1 macrophages (n = 4) and HepG2 hepatocytes (n = 3). Data are normalized on the number of platelet-binding cells with control platelets. (I) Platelets from P1 (red) (n = 3) or healthy controls (green) (n = 3) were labeled with fluorophore conjugated anti-GPIbα Fab′-fragments (6B4). Fluorophore lifetime in presence and absence of an acceptor was assessed and used to calculate fluorescence resonance energy transfer efficiency. (J) Change in platelet count in P1 and P2 after initiation of treatment with romiplostim (black arrow).
All patients provided informed consent in accordance with the declaration of Helsinki. P1 provided blood samples for additional analyses. Healthy subjects were recruited among employees and students of the UMC Utrecht. Institutional ethics review board approval was obtained, and all blood donors gave written informed consent.
111In-labeled platelet scanning was performed as described.9 Platelet signals in spleen and liver were quantified at 30 minutes, 3 hours, 24 hours, and 48 hours. The combined signals of liver and spleen were set at 100% and relative sequestration of radioactive platelets to liver or spleen was assessed as described.10 A spleen:liver ratio <0.8 indicates hepatic sequestration. Radioactivity in blood samples acquired at these time points was used to assess clearance rates, with radioactivity at 30 minutes set at 100%.
Platelet reactivity was determined as described.11 For measurement of surface glycans, whole blood or human embryonic kidney (HEK)293 cells were labeled with either fluorescein-conjugated ricinus communis agglutinin (RCA)-1 (Vector Labs, Burlingame, CA) or fluorescein-conjugated Sambucus nigra lectin (SNA) (Vector Labs), fixated and analyzed on a BD FACS Canto II (BD Biosciences, San Jose, CA). Platelets and cells were identified based on forward and side scatter. Glycan exposure on platelets and cells was expressed as median fluorescent intensity.
A guide RNA targeting exon 2 of the GNE gene (5′-AAACCGATCATGTTTGGCATTAAAC-3′) was cloned into the pSpCas9(BB)-2A-Puro (PX459) V2.0 plasmid, a gift from Professor Feng Zhang (Addgene plasmid #62988), as previously described.12 HEK293 cells were transfected with PX459-GNE plasmid. GNE knockout cells were selected with puromycin and transfected with pcDNA6 containing either wild-type (WT) GNE (based on NM_005476.6) or the Q420-GNE variant. GNE overexpressing cells were selected with blasticidin.
Washed platelets were obtained as previously described.13 Platelets were labeled with CellTracker Deep Red (Invitrogen, Carlsbad, CA). Human hepatocarcinoma (HepG2) cells were seeded and cultured for 24 hours in a 24-well plate. Human mononuclear THP-1 cells were seeded and differentiated into macrophages with 0.1 µM PMA for 24 hours. Serum-free medium was added to HepG2 cells or THP-1 macrophages and incubated for 30 minutes before adding labeled platelets. Platelets were incubated for 30 minutes, unbound platelets were washed away, and cells were harvested and analyzed with flow cytometry. HepG2 or THP-1 cells were gated based on forward and sideward scatter. Platelet binding was defined as the percentage Celltracker-positive HepG2 or THP-1 cells.
GPIbα clustering was measured as described.14 Fluorescence resonance energy transfer efficiency was used as a measure of GPIbα clustering and was defined as: , with τ as the lifetime of the donor fluorophore in the absence () or presence) of the acceptor.
Statistical analyses were performed in GraphPad Prism 9 (San Diego, CA). Differences between conditions were analyzed with Student t tests. P values < .05 were considered statistically significant.
The 420Q-GNE variant affects the ATP-binding region of the N-acetylmannosamine kinase domain of GNE and is predicted to disrupt phosphorylation of N-acetylmannosamine. To investigate functional consequences of the 420Q-GNE variant, both WT-GNE and Q420-GNE were introduced in engineered GNE-deficient HEK293 cells. Compared with WT cells, GNE-deficient cells had reduced sialic acid (P = .01) and increased galactose surface expression (P = .01) (Figure 1D). Overexpression of WT-GNE fully restored the glycosylation profile but overexpression of 420Q-GNE did not, confirming pathogenicity of the 420Q-GNE variant.
Both patients had normal glycosylation of transferrin and APOCIII, accepted markers for N- and O-linked glycosylation, respectively15 (Table 1), similar to what was reported in GNE-related myopathy. Because both patients had severe macrothrombocytopenia, and platelet hyposialylation has been reported to induce platelet clearance,1 platelet glycosylation profiles were investigated (Figure 1E). Binding of SNA, which recognizes terminal sialic acid in α6-galactose linkage on N-linked glycans,16 was normal. In contrast, binding of RCA-1 was strongly increased. Because RCA-1 recognizes terminal galactose on both N- and O-linked glycans with or without sialic acid in α6 linkage but does not recognize galactose with sialic acid in α3-linkage,17 these data suggest reduced expression of sialic acid in α3 linkage with galactose. Sialic acid in α3 linkage with galactose is predominantly found on O-linked glycans. Interestingly, abnormal O-linked glycosylation has been reported in GNE myopathy.18
111In-labeled autologous platelet scanning was performed in P1, which confirmed enhanced platelet clearance and showed a very short platelet half-life of 16 hours (Figure 1F). In addition, there was hepatic sequestration of platelets, based on a spleen:liver ratio of 0.33 (Figure 1G). Consistent with clearance, rather than decreased production as the cause of the macrothrombocytopenia, both patients had normal TPO levels (Table 1). Hepatic clearance of desialylated platelets has been attributed to both hepatocytes and liver-resident macrophages.19,20 In line with these reports, platelets from P1 showed increased binding to both THP-1 macrophages and HepG2 cells (Figure 1H). Platelet GPIbα contains abundant N- and O-linked glycosylation sites. We have previously reported that platelet desialylation results in clustering of GPIbα, which leads to enhanced platelet clearance.14 Indeed, platelets from P1 showed substantial GPIbα clustering (Figure 1I).
To increase platelet production, P1 and P2 were treated with TPO-receptor agonist romiplostim (Figure 1J). Although this caused an increase in platelet count and ameliorated bleeding symptoms substantially, the platelet count remained low and platelets remained giant sized.
In conclusion, data presented here show that platelets from patients with the 420Q-GNE variant are hyposialylated and show GPIbα clustering. The hyposialylated platelets are rapidly cleared in the liver, leading to thrombocytopenia. Bleeding symptoms from thrombocytopenia can be successfully treated with romiplostim. Further mechanistic studies are required to investigate the association between platelet hyposialylation and GPIbα clustering in GNE macrothrombocytopenia, to unravel why platelets are giant sized in GNE macrothrombocytopenia and why only platelets seem affected by the 420Q-GNE variant.
Acknowledgments: Funding support for this article was provided by the Netherlands Thrombosis Foundation (2018-03).
Contribution: R.E.G.S. and R.T.U. designed the study; T.N., I.v.A., A.J.L., C.A.K., K.Y.H., S.A.E.S., A.H., D.J.v.d.H., J.J.v.d.S., and M.E.v.G. collected the data; T.N., I.v.A., R.E.G.S., A.H., and R.T.U. analyzed the data; and T.N., I.v.A., A.H., H.C.G., S.J.A.K., M.B., J.J.v.d.S., M.E.v.G., and R.T.U. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rolf T. Urbanus, Center for Benign Hematology, Thrombosis and Hemostasis, Van Creveldkliniek, University Medical Center Utrecht, room C01.428, P.O. BOX 85500, 3508 GA Utrecht, The Netherlands; e-mail: r.t.urbanus@umcutrecht.nl.
References
Author notes
T.N. and I.v.A. contributed equally to this study.
Requests for data sharing may be submitted to Rolf T. Urbanus (r.t.urbanus@umcutrecht.nl).