Key Points
Higher axi-cel blood peak concentrations are associated with better response rates and longer PFS.
Abstract
Data on the association between chimeric antigen receptor (CAR)-T-cell kinetics and patient outcome in the nontrial setting are missing, mainly due to the lack of broadly available CAR-T-cell diagnostic quantification tools. We performed prospective quantification of axicabtagene ciloleucel (axi-cel) in 21 patients treated for aggressive B-cell lymphoma at our clinic. Median peak CAR-T-cell count was 16.14 CAR-T cells/µL. Patients with 16.14/μL or higher peak CAR-T cells (strong expanders) had more day-30 objective responses (91% vs 40%, P = .02). In univariate analysis, peak CAR-T cell ≥ 16.14 (P < .001), normal platelet counts at start of lymphodepletion (P < .001), no prior stem cell transplant (P = .04), and peak CAR-T cells as continuous variable (P = .03) were associated with better progression-free survival (PFS). After adjusting for platelet counts and prior stem cell transplantation, peak CAR-T cells below median was still associated with shorter PFS (relative risk, 0.15, 95% confidence interval, 0.04-0.59, P = .007). Low platelet counts also maintained significant impact on PFS. Our data demonstrate association of axi-cel levels and outcome in a nontrial setting and for the first time use a cutoff to segregate weak and strong expanders with respective outcomes.
Introduction
Chimeric antigen receptor (CAR)-T-cell products (tisagenlecleucel/Kymriah and axicabtagene ciloleucel [axi-cel]/Yescarta) have been approved for treatment of relapsed and/or refractory (r/r) B-cell malignancies.1,2 CAR-T-cell engraftment and expansion are thought to represent crucial parameters for treatment outcome. However, data correlating outcome and persistence in the nontrial setting are scarce, mainly due to limited availability of CAR-T-cell detection and quantification tools in the respective medical centers. Data from the ZUMA-1 trial show an association between peak concentrations of CAR-T cells and response.1,3 Real-world data from 2 multicenter retrospective analyses confirmed the efficacy and safety of axi-cel in a standard-of-care setting but did not include data on CAR-T-cell kinetics.4,5 We recently established and published methods for digital polymerase chain reaction (dPCR)-based quantification of CAR-T cells.6,7 We now study the impact of axi-cel CAR-T-cell blood concentrations on treatment outcome in real-world patients.
Methods
We performed prospective serial measurements of CAR-T-cell blood levels (for a detailed schedule, see supplemental Methods) of patients with r/r aggressive B-cell lymphoma (Table 1) treated with commercial axi-cel. The study was approved by the Hamburg General Medical Council ethics committee (PV7091) and written informed consent obtained from all patients. Patients received axi-cel (1-2 × 106 CAR-T cells/kg) after standard (n = 20) lymphodepletion with cyclophosphamide/fludarabine. Quantification of CAR-T cells was performed using a newly developed axi-cel-specific dPCR assay as described previously6 (and in supplemental Methods). Response assessment was performed (at 1, 3, 6, and 12 months) per institutional practice and based on Lugano criteria.8 Cytokine release syndrome (CRS) and immune effector cell associated neurotoxicity syndrome (ICANS) were defined and graded according to American Society for Transplantation and Cellular Therapy guidelines.9 The peak CAR-T-cell blood concentrations (CAR-T-Cmax) and the cumulative CAR-T-cell levels over the first 28 to 31 days in the peripheral blood by area under the curve (CAR-T-AUC) were analyzed using GraphPad PRISM Software (GraphPad Software). Statistical analyses were performed using SPSS Software (IBM, Germany).
Results and discussion
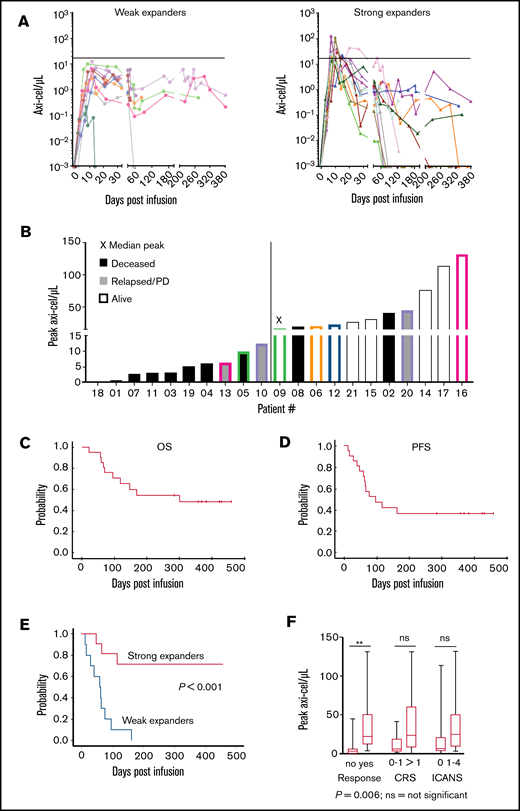
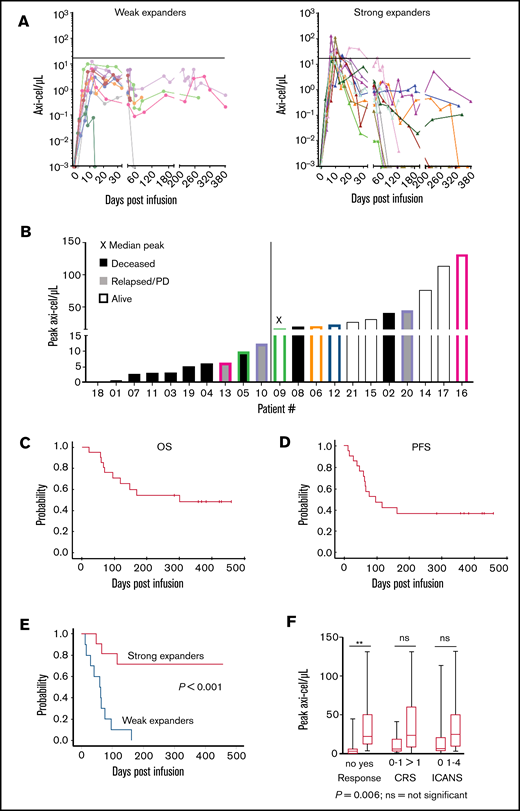
We included 22 consecutive, mostly heavily pretreated patients with r/r DLBCL or PMBCL (Table 1) who were apheresed and planned to receive axi-cel in a nontrial setting between March 2019 and July 2020 at our center. One patient was not infused due to disease progression; 21 of 22 patients received CAR-T-cells and were analyzed for outcome. CAR-T cells were detected in blood of all patients with similar kinetics as reported for ZUMA-1 (Figure 1A-B).1
“Real-world” axi-cel kinetics in 21 r/r B-NHL patients treated in our clinic. Analysis of peripheral blood mononuclear cells using an axi-cel–specific dPCR assay reveals differences in the CAR-T-cell engraftment kinetics. In vivo persistence of axi-cel T cells in the peripheral blood over time. The horizontal line indicates the median of the peak expansion. Negative values were set to 0.001/µL (limit of detection). (A) Strong expanders had a peak expansion of ≥16.14 cells/µL. (B) Peak expansion values and clinical outcome for individual patients. The median peak value (16.14 CAR T cells/µL) was found for patient 9. The color coding highlights the patients with prolonged persistence in each group. PD, progressive disease. (C-D) Kaplan-Meier curves of 21 patients treated with commercially available axi-cel in the nontrial setting show the OS and PFS during the >1 year of follow-up. (E) Kaplan-Meier curves of 21 axi-cel–treated patients show significantly increased survival of patients in the “strong expanders” group vs the “weak expanders” group. P value compares strong expanders vs weak expanders. (F) Boxplots showing CAR-T cell peak concentrations and correlation with response, CRS, and ICANS. The upper and lower borders of the box represent 25th and 75th percentiles, the line within the box depicts the median, and the bars represent the range. ns, nonsignificant.
“Real-world” axi-cel kinetics in 21 r/r B-NHL patients treated in our clinic. Analysis of peripheral blood mononuclear cells using an axi-cel–specific dPCR assay reveals differences in the CAR-T-cell engraftment kinetics. In vivo persistence of axi-cel T cells in the peripheral blood over time. The horizontal line indicates the median of the peak expansion. Negative values were set to 0.001/µL (limit of detection). (A) Strong expanders had a peak expansion of ≥16.14 cells/µL. (B) Peak expansion values and clinical outcome for individual patients. The median peak value (16.14 CAR T cells/µL) was found for patient 9. The color coding highlights the patients with prolonged persistence in each group. PD, progressive disease. (C-D) Kaplan-Meier curves of 21 patients treated with commercially available axi-cel in the nontrial setting show the OS and PFS during the >1 year of follow-up. (E) Kaplan-Meier curves of 21 axi-cel–treated patients show significantly increased survival of patients in the “strong expanders” group vs the “weak expanders” group. P value compares strong expanders vs weak expanders. (F) Boxplots showing CAR-T cell peak concentrations and correlation with response, CRS, and ICANS. The upper and lower borders of the box represent 25th and 75th percentiles, the line within the box depicts the median, and the bars represent the range. ns, nonsignificant.
CAR-T-Cmax ranged from 0.21 to 131.70 (median, 16.14) CAR-T cells/µL (Figure 1A-B). At 6 months, CAR-T cells could be detected in blood of 8 out of 10 evaluable patients with a median of 0.50 (range, 0.02-1.91) CAR-T cells/µL. CAR-T cells were also detectable in the blood of 3 out of 7 patients with ongoing remission at 10 to 12 months after treatment (Figure 1A-B). Patients with below-median CAR-T-Cmax were considered weak expanders, otherwise strong expanders. All patients were alive beyond 30 days and evaluable for response. Objective responses (partial or complete) around day 30 were observed in 14 out of 21 patients (67%), including 10 out of 11 strong expanders (91%) compared with 4 out of 10 weak expanders (40%) (P = .02).
After a median follow-up of 121 days (range, 22-379 days), 1-year estimates of overall survival (OS) and progression-free survival (PFS) were 49% (25% to 73%) and 37% (15% to 59%) for the entire cohort (Figure 1C-D). All 10 weak expanders either died due to disease progression (n = 8) or required other lymphoma therapy (n = 2). On the contrary, 9 out of 11 strong expanders are alive, including 8 without progression (Figure 1B). PFS at 1 year was 71% vs 0% (P < .001) for strong and weak expanders, respectively (Figure 1E). Median CAR-T-Cmax was higher in patients with complete or partial responses compared with nonresponders (22.06/μL [range, 3.20-131.7/μL] vs 3.02/μL [range, 0.21-44.80/μL], P = .006) (Figure 1F).
In univariate analysis, only strong expanders (P < .001), platelet counts >150/nL at apheresis (P < .001) or lymphodepletion (P < .001), no prior stem cell transplant (P = .04), and CAR-T-Cmax cells as a continuous variable (P = .03) were associated with better PFS. In Cox multiple regression models including 2 variables each (due to limited number of events), weak expanders still had lower PFS after adjusting for prior stem cell transplantation (relative risk [RR], 0.15; 95% confidence interval [CI], 0.04-0.62; P = .009), IPI (RR, 0.09; 95% CI, 0.02-0.47; P = .004), platelet counts (RR, 0.21; 95% CI, 0.05-0.86; P = .03), ICANS (RR, 0.13; 95% CI, 0.03-0.57; P = .001), and number of prior therapies (RR, 0.04; 95% CI, 0.007-0.25; P < .001), respectively. Among the other variables, only low platelet counts maintained significant impact on PFS after adjusting for CAR-T-Cmax (RR, 0.19; 95% CI, 0.05-0.86; P = .02). PFS at 1 year was 100% for patients with both high platelets and strong CAR-T-cell expansion (n = 8) and 0% for 5 patients with only 1 of the 2 (n = 3 strong expanders, n = 2 high platelets) or none of the 2 variables (n = 8).
Median AUC of CAR-T cells within 28 to 31 days was 138.4 (212.4-714.8). AUC above median was also associated with better PFS (P = .04). All other parameters listed in the patient demographics were not associated with response or PFS. Reasons for this could be that only 2 out of 21 patients did not require bridging therapy, and only 3 patients had an Eastern Cooperative Oncology Group score of ≥2. Due to impact of platelet counts, we also examined time from last chemotherapy and bone marrow involvement but found no association with response, expansion, or survival.
CRS was diagnosed in 15 patients (n = 2/10/3 for grades 1/2/3, respectively) at a median of 5 (0-11) days after CAR-T-cell infusion. ICANS was diagnosed in 10 patients (n = 5/1/3/1 for grades 1/2/3/4, respectively) after a median of 9 (3-10) days after CAR-T-cell infusion. Median CAR-T-Cmax also tended to be higher for patients with CRS 2-4 (n = 13) compared with those patients with CRS 0-1 (n = 8) (23.2 vs 5.8/μL, P = .07; Figure 1F). Likewise, median CAR-T-Cmax tended to be higher for 10 patients with compared with 11 patients without ICANS (25.0 vs 6.3/μL, P = .09; Figure 1F).
Three strong expanders required steroid treatment of ICANS and all are in ongoing remission at a median of 365 days after CAR-T-cell treatment.
To the best of our knowledge, our study represents the first nontrial data on association of CAR-T-cell kinetics and outcome of patients treated with axi-cel. Only 2 out of 21 patients met ZUMA-1 criteria, and both are alive progression-free. Patients were more heavily pretreated compared with other nontrial patients,5 as exemplified by a median of 5 (vs 3) lines of prior therapy, 62% (vs 35%) prior stem cell transplant, and 90% (vs 43%) not eligible for ZUMA-1. The 1-year PFS of 37% for our cohort is within the range of that reported for ZUMA-1 and nontrial patients. The median CAR-T-cell peak value of 16.14/μL is within the range of the data from the ZUMA-1 trial, with ∼40/μL for responders and ∼10/μL for nonresponders.1 We chose the median peak to avoid more arbitrary selection of a cutoff. Though both are quantitative PCR–based assays, the assay we recently developed and used in our study is not the same one used in the ZUMA-1 trial. We, for the first time, apply a cutoff to define strong vs weak expanders in a nontrial setting. We also find association of platelet counts <150/nL with poorer PFS. Low platelet count was not associated with prior autologous transplant. Factors such as bridging therapy, time from last chemotherapy to CAR-T-cell infusion, or bone marrow involvement were not associated with platelet count or PFS (supplemental Table 1). Our findings are restricted to axi-cel and cannot be generalized to other CAR-T-cell products. They are also limited by the small patient number and therefore cannot be considered conclusive. If confirmed in future studies, they may add to the available tools to be used to prognosticate patient outcome.
Acknowledgments
The authors are indebted to the teams of the Department of Stem Cell Transplantation, the Intensive Care Unit, and the Department of Neurology at our University Medical Center for excellent patient care. They also thank Michael Berger and our team of the Research Department Cell and Gene Therapy for technical help, support with sample analyses, and data processing. Finally, yet importantly, the authors wish to thank all patients for supporting this project.
This work was partly supported by a grant from the Barbara and Wilfried Mohr-Stiftung, (DM1669/100) (C.B. and B.F.). The funders had no role in the design of the study; the collection, analyses, or interpretation of data; the writing of the manuscript; or the decision to publish the results.
Authorship
Contribution: Patient treatment oversight was undertaken by F.A.A., N.K., D.W., C.F., G.T., and M.G.; conceptualization by F.A.A. and B.F.; methodology by C.B., A.B., and B.F.; data collection and formal analysis by C.B., A.B., F.A.A., B.F., M.G., T.S., T.Z., and S.Z.; interpretation by F.A., B.F., C.B., N.K., and K.R.; resources and/or research funding acquisition by N.K., B.F., and C.B.; visualization by C.B. and T.Z; supervision done by N.K., F.A.A., and B.F.; and project administration by F.A.A., C.B., and B.F.; the initial version of the manuscript was drafted by F.A.A. and B.F.; and all authors read and approved the final version of the manuscript.
Conflict-of-interest disclosure: F.A.A. received honoraria for participation in advisory boards from Kite/Gilead, Novartis, Celgene/BMS, Janssen, and Takeda. The dPCR assay described in this work has been made available as an “Expert Design Assay” (catalog number dEXD45718942) based on agreement between our medical center (Hamburg-Eppendorf) and Bio-Rad Laboratories (Hercules, CA). In accordance with German law on employee inventions, B.F., A.B., C.B., and K.R. participated in compensation payments. The remaining authors declare no competing financial interests.
ORCID profiles: K.R., 0000-0001-9050-6766; D.W., 0000-0002-4334-7640; N.K., 0000-0001-5103-9966; B.F., 0000-0001-9780-7211.
Correspondence: Francis A. Ayuk, Department of Stem Cell Transplantation, University Medical Center Hamburg-Eppendorf, Martinistr 52, 20246 Hamburg, Germany; e-mail: ayuketang@uke.de; and Boris Fehse, Department of Stem Cell Transplantation and Research Department Cell and Gene Therapy, University Medical Center Hamburg-Eppendorf, Martinistr 52, 20246 Hamburg, Germany; e-mail: fehse@uke.de.
References
Author notes
The full-text version of this article contains a data supplement.