Key Points
Treatment-free remission >3 years is seen in 30%-40% of FIP1L1-PDGFRA-positive patients who discontinued imatinib after achievement of CMR.
Abstract
FIP1L1-PDGFRA–positive myeloid/lymphoid neoplasms with eosinophilia (MLN-eo) are exquisitely sensitive to imatinib. Almost all patients achieve a complete molecular remission (CMR) by nested reverse transcription polymerase chain reaction, which can be maintained with low-dose imatinib (eg, 3 × 100 mg/wk). Because imatinib can be safely stopped in a substantial proportion of patients with BCR-ABL1–positive CML, we sought to analyze the clinical and molecular follow-up of 12 FIP1L1-PDGFRA–positive patients with MLN-eo in chronic phase who discontinued imatinib after achievement of a CMR. Median time of treatment and median time of CMR before imatinib discontinuation (last dose at 3 × 100 mg/wk, n = 8; or 100 mg/d, n = 4) were 80 (range, 43-175) and 66 (range, 37-174) months, respectively. A molecular relapse was observed in 4 patients after 10, 22 (n = 2), and 24 months. A second CMR was achieved in 3 patients after 3, 4, and 21 months. Eight patients (62%) are in ongoing CMR (median, 17 months; range, 3-71 months). Molecular relapse-free survival was 91% at 12 months and 65% at 24 months. No significant differences (eg, dose and duration of imatinib treatment or duration of CMR before imatinib discontinuation) were identified between patients with and without molecular relapse. Our data demonstrate that imatinib can be safely stopped in FIP1L1-PDGFRA–positive MLN-eo because of a high treatment-free remission at 12 and 24 months and because most patients achieve a rapid second CMR after restart of imatinib.
Introduction
The World Health Organization 2016 classification of myeloid neoplasms defines a distinct subgroup as “myeloid/lymphoid neoplasms with eosinophilia and rearrangement of PDGFRA, PDGFRB, FGFR1, or PCM1-JAK2 fusion gene” (MLN-eo). FIP1L1-PDGFRA, which is generated by a cytogenetically cryptic interstitial deletion on chromosome 4q12, is by far the most frequent fusion gene within this subgroup. The vast majority of patients are diagnosed in chronic phase; however, FIP1L1-PDGFRA can rarely also be identified in patients who present in blast phase of either myeloid (eg, acute myeloid leukemia, myeloid sarcoma) or lymphoid (eg, concurrently diagnosed T-lymphoblastic lymphoma) origin.1 More than 90% to 95% of chronic phase and blast phase patients achieve rapid and durable clinical, complete hematological remissions (CHR) and complete molecular remissions (CMR), as revealed by nested reverse transcription polymerase chain reaction (RT-PCR), when receiving monotherapy with low-dose (eg, 100 mg/d) imatinib.1 In the long-term, CMR can be maintained with 1 to 3 × 100 mg/wk,2 and the 5-year survival rate for patients in chronic phase is more than 90%.3 Analogous to modern management of chronic myeloid leukemia (CML),4,5 hematologists and patients frequently ask for the option to safely stop imatinib once they have achieved sustained CMR.2,6-9
Methods
We retrospectively analyzed the clinical, hematological, and molecular follow-up of a series of FIP1L1-PDGFRA–positive patients who discontinued imatinib after variable intervals from achievement of a CMR. As previously described,10 nested RT-PCR (sensitivity of 10−3 to 10−4) for the detection of the FIP1L1-PDGFRA fusion gene and monitoring of CMR was performed on RNA extracted from peripheral blood samples. As this was a registry study, there was no strict plan for molecular monitoring. In the absence of increase of eosinophils, we recommended performing RT-PCR every 3 months in the first 2 years and every 3 to 6 months thereafter. Within the German Registry on Disorders of Eosinophils and Mast Cells, we identified 76 patients receiving treatment with imatinib, of whom 12 (16%) patients had stopped imatinib therapy after achievement of CMR (all male; median age, 45 years; range, 27-70 years). The main reason for discontinuation of imatinib was the patients’ wish to stop (n = 11); for example, because of the positive results in BCR-ABL1–positive CML or because of planned pregnancy (n = 1). Fifty-two of the remaining 64 FIP1L1-PDGFRA–positive patients had at least 1 documented negative RT-PCR during follow-up and are in ongoing CMR for a median of 95 months (range, 1-173 months). With the exception of patients who initially presented in blast phase (n = 17), we could not identify any significant differences between the patients who stopped imatinib (n = 12, all in chronic phase) and the patients who continued imatinib (n = 64) regarding available baseline (eg, absolute or relative number of eosinophils, other peripheral blood counts, splenomegaly or bone marrow features) and treatment (eg, time of treatment or duration of CMR) characteristics.
Results and discussion
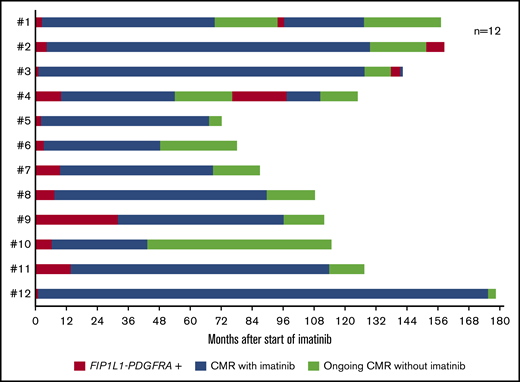
Median time on imatinib was 80 (range, 43-175) months, and median time in CMR was 66 (range, 37-174) months. Before discontinuation, 4 patients were receiving 100 mg imatinib per day and 8 patients were receiving 3 × 100 mg/wk (Figure 1). A molecular relapse was observed in 4 patients after 10, 22 (n = 2), and 24 months, but no patient had a hematological relapse. In those 4 patients, median time receiving imatinib was 98 (range 54-129) months, and median time in CMR was 96 (range 44-126) months. The imatinib dose before discontinuation in these 4 patients was 3 × 100 mg/wk (n = 3) and 100 mg/d (n = 1). On imatinib 100 mg/d, 3 patients achieved a second CMR after 3, 4, and 21 months; the fourth patient remains nested RT-PCR positive at 7 months without detection of a PDGFRA mutation conferring resistance, such as PDGFRA T674I or D842V. Eight patients (62%) are in ongoing CMR (median, 17 months; range, 3-71 months; 4/8 [50%] patients >12 months, 2/8 [25%] patients >24 months). Molecular relapse-free survival was 91% at 12 months and 65% at 24 months; median relapse-free survival was 24 months (Figure 2A). No significant differences (eg, imatinib dose, median duration of treatment, or median time of CMR before imatinib discontinuation) were identified between patients with and without molecular relapse.
Follow-up of 12 patients with FIP1L1-PDGFRA–positive MLN-eo after discontinuation of imatinib. CMR was assessed by nested RT-PCR.
Follow-up of 12 patients with FIP1L1-PDGFRA–positive MLN-eo after discontinuation of imatinib. CMR was assessed by nested RT-PCR.
Relapse-free survival in FIP1L1-PDGFRA–positive MLN-eo after discontinuation of imatinib. (A) Kaplan-Meier estimates for loss of CMR after discontinuation of imatinib in 12 patients with FIP1L1-PDGFRA–positive MLN-eo. (B) Kaplan-Meier estimates for loss of CHR/CMR (n = 33) or loss of CMR only (n = 25) after discontinuation of imatinib in 33 patients with FIP1L1-PDGFRA–positive MLN-eo from this series (n = 12) and an additional 19 cases reported in the literature.
Relapse-free survival in FIP1L1-PDGFRA–positive MLN-eo after discontinuation of imatinib. (A) Kaplan-Meier estimates for loss of CMR after discontinuation of imatinib in 12 patients with FIP1L1-PDGFRA–positive MLN-eo. (B) Kaplan-Meier estimates for loss of CHR/CMR (n = 33) or loss of CMR only (n = 25) after discontinuation of imatinib in 33 patients with FIP1L1-PDGFRA–positive MLN-eo from this series (n = 12) and an additional 19 cases reported in the literature.
In the first reported series, imatinib was discontinued in 4/5 FIP1L1-PDGFRA–positive patients after a median of 25.5 months, and all patients experienced a molecular relapse within 6 months.7 Legrand et al described the discontinuation of imatinib after median of 30.2 months in CHR (n = 11) and/or CMR (n = 8), respectively.2 A hematological or molecular relapse was observed in 6/11 patients within 1 to 27 months, and 5 patients remained in CHR (n = 1) or CMR (n = 4) for a median of 31 months. Relapse-free survival was 61% at 12 months and 42% at 24 months. Helbig et al discontinued imatinib in 7 patients after a median of 79.2 months receiving imatinib and a median of 55.2 months in CMR.6 Three patients experienced a molecular relapse within 6 months, and 4 patients remained in CMR for a median of 28 months. Relapse-free survival was 57% at 12 months. In the most recent report, Qu et al reported on 8 patients with hematological relapse within 2 to 48 months in 4 patients and durable CHR for median of 47 months in the 4 other patients, 2 of them in CMR.9 Two patients experienced secondary resistance to imatinib resulting from a PDGFRA T674I mutation with fatal outcome.
In total, imatinib discontinuation has been reported in 42 cases.2,6,7,9 Unfortunately, the heterogeneity of reported types of relapse as hematological relapse, molecular relapse, or a combination of both did not allow a clear separation between hematological and molecular relapse. Imatinib was discontinued after a median of 55 (range, 4-175) months in CHR and a median of 44 (range, 0.5-174) months in CMR. No tyrosine kinase inhibitor (TKI) withdrawal syndrome5 was reported. A hematological or molecular relapse occurred in 21/42 (50%) patients after a median of 5.6 (range, 1-48) months (Figure 2B). Twelve (71%) of 17 and 14/17 (82%) molecular relapses occurred within the first 12 and 24 months, respectively. Twenty-one patients have remained in CMR/CHR for median of 19 (range, 2-74) months. Relapse-free survival was 63% at 12 months and 50% at 24 months. Imatinib was reinitiated at the last effective dose in 19 patients, resulting in rapid reinduction of remission in 16/19 patients. Secondary resistance was reported in 2/42 patients.9 One patient was treated for 4.8 months only and developed a PDGFRA T674I mutation after imatinib discontinuation for 48 months, whereas the second patient presented with imatinib-resistant blast phase 2 months after discontinuation. Overall, death occurred in 3/42 patients (PDGFRA T674I mutation, n = 2; unknown cause, n = 1).9
In chronic phase BCR-ABL1–positive CML, data from large series have been reported on discontinuation of frontline TKI therapy.5,11-12 The best predictors for treatment-free remission after discontinuation are duration of treatment and duration of deep molecular response (MR4 or better, corresponding to BCR-ABL1 ≤ 0.01% on the International Scale). Moreover, the vast majority of patients who relapse can expect to regain remission shortly after reinitiation of the TKI.13 Overall, therefore, the clinical outcome after stopping therapy is strikingly similar for BCR-ABL1–positive CML and FIP1L1-PDGFRA–positive disease. In CML, considerable weight has been given to frequent molecular monitoring, both to define patients who are eligible for stopping and for early detection of relapse. Molecular monitoring is also important for FIP1L1-PDGFRA; however, the level of expression of the fusion gene at diagnosis is often low, and thus sensitivity of detection is only 10−3 to 10−4 for many cases,10 which is insufficient to define deep molecular response. This difference, as well as the relatively small number of cases, may explain why we failed to see an association between duration of CMR and outcome for FIP1L1-PDGFRA–positive MLN-eo. More sensitive methods based on quantification of genomic DNA fusion junctions may be of greater value in this context.14
We conclude that imatinib can be discontinued in FIP1L1-PDGFRA–positive MLN-eo after achievement of CMR, the median time to relapse is 5.6 months, there is no association between duration of treatment or duration of CMR and risk for relapse, treatment-free remission longer than 3 years is observed in approximately 30% to 40% of patients, and the vast majority of patients achieves a rapid second CHR/CMR after reinitiation of imatinib.
Acknowledgments
The authors thank all physicians who contributed clinical data of their patients into the German Registry on Disorders of Eosinophils and Mast Cells, and in particular Karin Bonatz, Susanne Simon-Becker, Regina Herbst, Ole Maywald, Dirk Medgenberg, Marion Schmalfeld, and Ellen Wollmer for this manuscript.
This work was supported by the “Deutsche José Carreras Leukämie-Stifung e.V.” (grant no. 01 R/2018).
Authorship
Contribution: G.M. and A.R. drafted the manuscript; and all authors contributed to the manuscript, provided major intellectual contributions, reviewed and revised its content, and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andreas Reiter, Department of Hematology and Oncology, University Hospital Mannheim, Heidelberg University, Theodor-Kutzer Ufer 1-3, 68167 Mannheim, Germany, andreas.reiter@medma.uni-heidelberg.de