Key Points
rhTPO could be an effective and safe treatment option for promoting platelet engraftment for hematological malignancies received UCBT.
Delayed platelet engraftment is a common complication after umbilical cord blood transplantation (UCBT) accompanied by increased transplant-related complications or death. This study was designed to determine the safety and efficacy of recombinant human thrombopoietin (rhTPO) in promoting platelet engraftment after UCBT. A total of 120 patients scheduled to receive UCBT were randomly assigned to the rhTPO group (300 U/kg once daily from days 14 to 28 after UCBT, n = 60) or the control group (n = 60). The primary outcome was the 60-day cumulative incidence of platelet engraftment after single-unit cord blood transplantation. The 60-day cumulative incidence of platelet engraftment (platelet count ≥20 × 109/L) and the 120-day cumulative incidence of platelet recovery (platelet count ≥50 × 109/L) were both significantly higher in the rhTPO group than in the control group (83.1% vs 66.7%, P = .020; and 81.4% vs 65.0%, P = .032, respectively). In addition, the number of required platelet infusions was significantly lower in the rhTPO group than in the control group (6 vs 8 units, respectively; P = .026). The cumulative incidence of neutrophil engraftment and the probability of 2-year overall survival, disease-free survival, and graft-versus-host disease–free relapse-free survival did not differ between the 2 groups. Other transplant-related outcomes and complications did not differ between the 2 groups, and no severe adverse effects were observed in patients receiving rhTPO. This study demonstrated that rhTPO is well tolerated in patients and could effectively promote platelet engraftment after UCBT. This study was registered on the Chinese Clinical Trial Registry (http://www.chictr.org.cn/index.aspx) as ChiCTR-IPR-16009357.
Introduction
Cord blood (CB) has been proven to be an effective and reliable alternative donor source for hematologic diseases.1-4 However, the utility of umbilical cord blood transplantation (UCBT) has been strongly restricted because of a lower number of hematopoietic progenitors in CB, which is accompanied by a higher incidence of prolonged hematopoietic recovery, especially delayed platelet engraftment (DPE). DPE can lead to more transplant-related complications or death and increased necessity of platelet transfusion.5-7
Aside from the diagnosis and disease status, prehematopoietic stem cell transplantation (HSCT), endogenous thrombopoietin (TPO) levels before conditioning, conditioning regimen, viral infection, and graft-versus-host disease (GVHD), CD34+ cells are generally accepted as the most important risk factors associated with DPE after HSCT and result in poor prognosis.7-10 Total nucleated cell dose, CD34+ cells and high-resolution HLA matching are the crucial prognostic factors in UCBT, but comprehensive analysis of many studies identified that once a certain threshold is reached, the increase in CD34+ cells does not accelerate platelet engraftment.10-12 Therefore, thrombocytopenia after cord blood transplantation may be caused by some other intrinsic factors. Studies demonstrated that the megakaryocytes (MKs) derived from CB are significantly smaller and of lower ploidy than adult MKs from mobilized bone marrow or peripheral blood.13-15 In addition, the attainment of adult size in CB-derived MKs is delayed after UCBT.16,17 Low ploidy of CB-derived MKs may contribute to the DPE after UCBT.18,19 Thrombocytopoiesis is regulated by multiple cytokines, and TPO has been identified as a key cytokine in the procedure.20 Therefore, TPO mimetics, which can promote megakaryocyte proliferation and platelet maturation, are expected to be potential therapeutic options for DPE after UCBT. Many studies have shown the safety and certain efficacy of TPO mimetics in treating thrombocytopenia after HSCT.21-23 However, there is a paucity of information regarding the management of prolonged thrombocytopenia after UCBT.
Recombinant human TPO (rhTPO) is a full-length glycosylated TPO recombinant protein produced in Chinese hamster ovary cells and purified by bioengineering techniques. The amino acid sequence of rhTPO is identical to that of endogenous TPO. Multicenter trials showed that rhTPO was effective and safe in the treatment of thrombocytopenia after allo-HSCT22,24 and various immune thrombocytopenia patients.25,26 Different from pegylated human recombinant megakaryocytic growth and development factor, no persistent neutralizing antibodies against rhTPO were detected in these studies.22,24-26 Therefore, we performed a single-center, prospective, and randomized study to investigate the safety and efficacy of rhTPO in patients receiving UCBT.
Methods
Patient eligibility
Between 20 October 2016 and 26 March 2018, a total of 120 consecutive subjects with hematologic malignancies scheduled to receive single unrelated UCBT were enrolled in the study. Eligibility criteria were as follows: (1) body weight ≥30 kg and age ≤60 years; (2) Karnofsky performance status must be ≥70%; (3) no HLA-compatible related donors were available (all subjects received single-unit UCBT but not double-unit UCBT); and (4) CB units chosen for transplantation from Chinese CB banks were serologically matched for ≥4 of 6 HLA antigens and contained at least 3.0 × 107/kg of recipient body weight total nucleated cells and 1.2 × 105/kg CD34+ cells before freezing.
Exclusion criteria were as follows: uncontrolled infection, history of thromboembolic disease in the previous 6 months, myelofibrosis and severe heart, liver, kidney, and lung dysfunction; receiving a previous autologous or allogeneic transplantation before; and developing severe adverse events (grade 4 or 5) defined by the Common Terminology Criteria for Adverse Events version 4.027 during the conditioning period. This study protocol was approved by the Ethics Committee of the First Affiliated Hospital of the University of Science and Technology of China. Informed consent was obtained in accordance with the Declaration of Helsinki. The trial was registered on the Chinese Clinical Trial Registry (ChiCTR-IPR-16009357).
Randomization and transplantation procedures
Eligible participants were randomly assigned (1:1) to the rhTPO group or the control group using a randomization table. Patients in the rhTPO group received a subcutaneous injection of rhTPO (Sansheng Pharmaceutical, Shenyang, China) at a dose of 300 U/kg (maximum dose 15 000 U) once daily from days 14 to 28 after UCBT. The subcutaneous injections were discontinued after platelet engraftment was achieved. In addition, if the platelet count did not recover to ≥20 × 109/L by day 28 after UCBT, the administration of rhTPO was discontinued. Patients in the control group did not receive rhTPO injections.
All patients received a myeloablative conditioning regimen, which consisted of a full dose of busulfan (BU, total 12.8 mg/kg, 0.8 mg/kg every 6 hours for 4 days), cyclophosphamide (CY, 60 mg/kg daily for 2 days) plus fludarabine (30 mg/m2 daily for 4 days) or total body irradiation (total 12 Gy, 4 fractions) and CY (60 mg/kg daily for 2 days) plus cytarabine (2 g/m2 twice for 2 days). The GVHD prophylaxis consisted of cyclosporine and mycophenolate mofetil without antithymocyte globulin. Granulocyte colony-stimulating factor (5-7 µg/kg per day) was started from day 6 until the neutrophil count was ≥0.5 × 109/L for 3 consecutive days. Other treatments were performed in accordance with the standard UCBT procedures according to published criteria.28 For sinusoidal obstruction syndrome or veno-occlusive disease prophylaxis, heparin was administered at a dose of 100 IU/kg per day until platelet count <20 × 109/L, and prostaglandin E1 (PGE1) was given at a dose of 0.03 μg/kg per hour through continuous infusion, 12 to 24 hours before the start of conditioning regimen and continued until day 30.
End points
The primary end points of this trial were the platelet engraftment time and the 60-day cumulative incidence of platelet engraftment after UCBT. The date of platelet engraftment was defined as the first day of platelet recovery to ≥20 × 109/L without transfusion support for 7 consecutive days.
The secondary end points included platelet recovery, the units of platelet transfusions, neutrophil engraftment, overall survival (OS), disease-free survival (DFS), GVHD-free, relapse-free survival (GRFS), incidence and severity of acute GVHD and chronic GVHD,18 transplantation-related mortality (TRM), and relapse rate. The date of platelet recovery was defined as the first day of platelet recovery to ≥50 × 109/L without transfusion support for 7 consecutive days. Platelet transfusion was performed when the platelet count was <10 × 109/L or in the event of significant bleeding risk, regardless of the platelet count, according to the clinical practice guidelines of the American Association of Blood Banks.29 The novel composite end point of GRFS was defined as grade 2 to 4 acute GVHD, chronic GVHD requiring systemic treatment, relapse, or death.30 The patients were stratified into 4 risk groups based on the disease type and status at the time of transplantation, known as the disease risk index.31
Safety
Safety analyses were performed based on the incidence of adverse events after UCBT, excluding those expected related to UCBT. Adverse events, including laboratory abnormalities and clinical symptoms relating to rhTPO, were recorded and graded according to the Common Terminology Criteria for Adverse Events version 4.0. All patients were assessed for the safety of the treatment at 4-week intervals for 24 weeks after the first dose of rhTPO.
The assessment of bleeding events graded as minor, major non–life-threatening, and major life-threatening, with the exception of mild petechiae, was performed during the first 60 days after UCBT according to the previously described bleeding scoring system.32 In previous studies, neutralizing antibodies targeting endogenous TPO were found in patients who received pegylated human recombinant megakaryocytic growth and development factor.33,34 No sustained anti-TPO antibodies were detected in participants treated with rhTPO.25,26 We routinely collected serum samples from the rhTPO group 4 weeks and 6 months after the first dose or at the end of rhTPO treatment to determine the presence of anti-TPO antibody using an enzyme-linked immunosorbent assay; if positive, samples were then tested using a neutralization assay.
Sample size calculation and statistical analysis
The primary objectives of the study were to compare the platelet engraftment time and the 60-day cumulative incidence of platelet engraftment between the 2 study arms. In our preliminary experiment, 20 patients (body weight ≥30 kg) with a hematologic malignancy were treated with rhTPO from days 14 to 28 after UCBT, whereas 20 patients (body weight ≥30 kg) were in the control group and not treated with rhTPO. We calculated the sample size using PASS software and noted that a total sample size of 120 subjects was needed to detect a decrease in the median platelet engraftment time from 39.3 ± 15.51 to 33 ± 4.2 days at an α level of 0.05 (2 sided) with 80% power and an assumed attrition rate of 15%.
The intent-to-treat analysis was conducted for the primary and secondary end points. The cumulative incidence of neutrophil engraftment, platelet engraftment, platelet recovery, 100-day acute GVHD, 2-year chronic GVHD, 2-year TRM, and relapse was compared between groups using Gray’s test. Continuous and ordinal variables were compared using the Mann-Whitney U test, and categorical variables were compared using Pearson's χ2 test. The probability of 2-year OS, DFS, and GRFS was estimated using the Kaplan-Meier method and compared between groups using the log-rank test.
Univariate and multivariate analyses of the factors associated with platelet engraftment were performed using the Cox proportional hazards regression model. First, a univariate analysis was performed for each factor. The factors with P < .10 in the univariate analysis were included in a multivariate regression analysis, and those with P < .05 in the multivariate analysis were considered to be significant. All statistical analyses were performed using SPSS 23.0 software (SPSS, Inc., Chicago, IL) and R software, and P < .05 (2 sided) was considered significant.
Results
Patients enrollment and follow-up
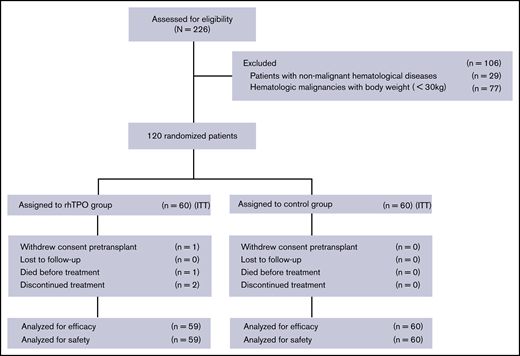
All surviving patients were followed until 29 February 2020, and the median follow-up time from UCBT was 943 days (range, 705-1227 days). Figure 1 shows the trial profile: 60 patients were randomized to receive rhTPO, and 60 were controls. One randomized patient withdrew consent and did not received a transplant. One patient died on day 13 before receiving rhTPO, and 2 patients discontinued rhTPO on days 18 and 21 because they refused to continue the rhTPO subcutaneous injection.
Trial profile. Flowchart of patients enrolled in the rhTPO group and the control group.
Trial profile. Flowchart of patients enrolled in the rhTPO group and the control group.
As shown in Table 1, the baseline demographics and clinical characteristics were balanced between the 2 groups. The median age was 26.5 years (range, 8-59 years), and the median weight was 57.5 kg (range, 30-91 kg). Fifty-two patients had acute myeloid leukemia, 37 patients had acute lymphocyte leukemia, 8 patients had chronic myeloid leukemia, 9 patients had myelodysplastic syndrome, 9 patients had lymphoma, 1 patient had plasma cell leukemia, 2 patients had mixed phenotype acute leukemia, and 1 patient had 8p11 myeloproliferative syndrome. The patients were stratified into low (n = 7), intermediate (n = 72), high (n = 35), and very high (n = 5) risk groups based on the disease risk index assignment. The mean interval from randomization to UCBT was 8 days (range, 7-15 days).
Response
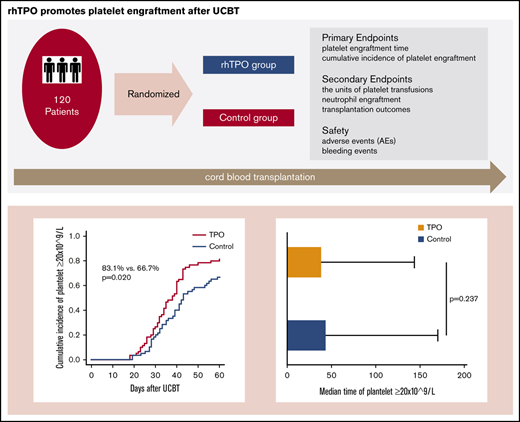
The 60-day cumulative incidence of platelet engraftment in the rhTPO group was 83.1% (95% confidence interval [CI], 70.3%-90.7%), which was significantly higher than that in the control group (66.7%; 95% CI, 53.0%-77.2%; P = .020; Figure 2A). However, the median platelet engraftment time was similar in both groups: 38 days (range, 18-144 days) in the rhTPO group and 43 days (range, 19-170 days) in the control group (P = .237; Figure 2C). Univariate analysis indicated that platelet engraftment was associated with the following factors: group (the rhTPO or control group; P = .035), conditioning regimen (P = .002), cell doses of infusion total nuclear cell (P = .026), and CD34+ cells (P = .004). In multivariate analysis, the results showed that in the rhTPO group (hazard ratio [HR] = 1.696; 95% CI, 1.108-2.597; P = .015), the BU-CY2–based conditioning regimen (HR = 4.082; 95% CI, 1.764-9.446; P = .001) and increasing numbers of infusion CD34+ cell doses (HR = 1.743; 95% CI, 1.122-2.708; P = .013) were independent predictors of platelet engraftment (Table 2). We also did the subanalysis in patients who received infused CD34+ cells < or ≥1.6 × 105/kg, respectively. The results showed that for patients who received infused CD34+ cells <1.6 × 105/kg, the 60-day cumulative incidence of platelet engraftment was significantly higher in the rhTPO group than in the control group (79.3%; 95% CI, 58.3%-90.5% vs 53.3%; 95% CI, 33.7%-69.5%; P = .013). However, for patients who received infused CD34+ cells ≥1.6 × 105/kg, the 60-day cumulative incidence of platelet engraftment was a little higher in the rhTPO group than in the control group but had no statistical difference (86.7%; 95% CI, 66.2%-95.2% vs 80.0%; 95% CI, 59.4%-90.9%; P = .441).
Platelet engraftment, median time of platelet engraftment, platelet recovery, and numbers of required platelet transfusion in the rhTPO group (n = 59) and the control group (n = 60). (A) Sixty-day cumulative incidence of platelet ≥20 × 109/L between the rhTPO group and control group. (B) One hundred twenty–day cumulative incidence of platelet ≥50 × 109/L between the rhTPO group and control group. (C) Median time of platelet ≥20 × 109/L in the rhTPO group and control group. (D) Numbers of required platelet transfusion in the rhTPO group and control group.
Platelet engraftment, median time of platelet engraftment, platelet recovery, and numbers of required platelet transfusion in the rhTPO group (n = 59) and the control group (n = 60). (A) Sixty-day cumulative incidence of platelet ≥20 × 109/L between the rhTPO group and control group. (B) One hundred twenty–day cumulative incidence of platelet ≥50 × 109/L between the rhTPO group and control group. (C) Median time of platelet ≥20 × 109/L in the rhTPO group and control group. (D) Numbers of required platelet transfusion in the rhTPO group and control group.
The 120-day cumulative incidence of platelet recovery was 81.4% (95% CI, 68.3%-89.4%) in the rhTPO group and 65.0% (95% CI, 51.3%-75.7%) in the control group (P = .032; Figure 2B). In addition, the platelet recovery time in the rhTPO and control groups were 43 days (range, 25-171 days) and 53 days (range, 28-198 days), respectively (P = .053).
The number of required platelet transfusions was 6 units (range, 0-21 units) and 8 units (range, 2-28 units; P = .026) within 60 days after UCBT in the rhTPO and control groups, respectively, and the number of required platelet transfusions from days 14 to 60 was significantly lower in the rhTPO group (3 units; range, 0-19 units) than in the control group (4.5 units; range, 0-25 units; P = .035; Figure 2D).
Transplantation outcomes
The 42-day cumulative incidence of neutrophil engraftment and the median neutrophil engraftment time did not differ between the 2 groups: 98.3% (95% CI, 75.6%-99.9%) and 17.5 days (range, 11-29 days) in the rhTPO group and 95.0% (95% CI, 84.1%-98.59%) and 17 days (range, 12-32 days) in the control group (P = .148 and P = .924, respectively; Table 3).
Other post-transplant outcomes are provided in Table 3. The 2-year cumulative incidence of relapse was similar between the rhTPO group at 13.6% (95% CI, 6.3%-23.7%) and the control group at 20.0% (95% CI, 10.9%-31.0%; P = .298). The probabilities of 2-year OS, DFS, and GRFS were not significantly different between the 2 groups (64.4%; 95% CI, 50.8%-75.1%; 55.9%; 95% CI, 42.4%-67.5%; and 45.8%; 95% CI, 32.8%-57.8% in the rhTPO group vs 51.7%; 95% CI, 38.4%-63.4%; 50.0%; 95% CI, 36.8%-61.8%; and 41.7%; 95% CI, 29.2%-53.7% in the control group, respectively). Other transplant-related outcomes, such as the cumulative incidences of TRM, pre-engraftment syndrome, grade 2 to 4 and grade 3 to 4 acute GVHD, chronic GVHD, and incidence of infection, did not differ between the 2 groups (Table 3).
Safety and tolerability
Safety and adverse events were evaluated in 119 participants. The adverse event leading to treatment discontinuation was pain at the injection site, which was observed in 2 patients. Laboratory abnormalities involving hepatic function, renal function, or coagulative function did not differ between the rhTPO group and the control group (supplemental Table 1). Clinical symptoms reported in previous studies to be associated with the use of rhTPO or other TPO mimetics21-26 also did not differ between the 2 groups (supplemental Table 1). All grade 3 to 4 adverse events were deemed by the investigator as unlikely to be related to rhTPO treatment.
The bleeding events during the first 60 days after UCBT according to the predefined bleeding severity grade showed no differences between the 2 groups (P > .05; supplemental Table 2). Transplant-related complications, such as incidence of bloodstream infection, fungal infection (proven and probable), and cytomegalovirus viremia also show similar results between the 2 groups (Table 3). Without regular bone marrow biopsy examination after UCBT, myelofibrosis was not evaluated in our study. No thromboembolic events occurred, and no neutralizing serum anti-TPO antibody was detected in all the participants.
Discussion
Despite the advantages of no harm to donors, less stringent HLA match, stronger graft-versus-leukemia effect, and lower incidence of chronic GVHD,1,35,36 clinical application of UCBT is still limited because of limited numbers of total nuclear cells and CD34+ cells in CB. Failed or delayed platelet engraftment is more common in UCBT than in other types of allo-HSCT, which necessitates platelet transfusion and leads to increased TRM and decreased survival.6,7 Recognizing that failed or delayed platelet engraftment restricts the clinical application of UCBT because of lower cell numbers, many strategies such as transplant with ex vivo–expanded CB or cocultured CB with mesenchymal stromal cells, cotransplant with a third haploidentical CD34+ stem cell, or direct intrabone CB transplantation have been used to overcome the limited number of CB cells and the consequent prolonged hematopoietic recovery.37-41 However, none of these methods seems to achieve reliable results or could be widely used. There is still no consensus for the management of prolonged post-UCBT thrombocytopenia, and platelet transfusion remains a mainstay of therapy.
Previous studies found that MK ploidy and endogenous TPO levels may be associated with DPE and have prognostic significance to allo-HSCT.9,14,18 It is well established that TPO promotes the proliferation of megakaryocyte progenitors and increases the ploidy of MKs signaling through its receptor Mpl, suggesting that TPO may play a critical role in both megakaryogenesis and thrombopoiesis after allo-HSCT.20,42 The development of TPO mimetic agents for treating DPE has evolved preliminary with good tolerance and certain availability in recent years.21-23 Because of conditioning regimen and gastrointestinal GVHD, patients undergoing HSCT often have nausea, vomiting, and diarrhea, affecting the absorption of oral drugs. Subcutaneous injection of TPO mimetics, such as rhTPO or romiplostim, may be superior to the oral form of eltrombopag.
In a phase 1/2 multicenter single-arm open trial, romiplostim was notably well tolerated in patients with transfusion-dependent thrombocytopenia after allogeneic HSCT and successfully improved platelet count to 50 × 109/L at a median of 45 days.21 Many other studies have achieved similar results using rhTPO or eltrombopag in the treatment of DPE.22-24 However, most of these studies were retrospective studies, and sample sizes were relatively sparse. In addition, limited to a nonrandomized controlled design, the variety of TPO mimetics, and the difference of in initial time, dosage, and duration of application, we cannot identify whether the platelet recovery was caused by TPO mimetics. After all, platelet count may spontaneously improve in many of these DPE patients even without any treatment. Although TPO mimetics have shown promising prospects for DPE, further randomized prospective phase 3 clinical trials are needed to confirm efficacy. There is no literature assessing whether TPO mimetics promote platelet engraftment in hematologic malignancy patients after UCBT. To the best of our knowledge, this report describes the first prospective, randomized study to investigate the safety and efficacy of rhTPO in a UCBT setting. The results from our prospective randomized controlled trial showed an increase in cumulative incidence of platelet engraftment and a reduction in median platelet engraftment and recovery times. Additionally, the number of required platelet transfusions from days 14 to 60 was significantly lower in the rhTPO group.
This present study indicated that rhTPO was well tolerated in patients after UCBT. The most common adverse events leading to discontinue were intolerance to the subcutaneous injections. Other most frequently adverse events, such as fever, diarrhea, fatigue, infection, and laboratory abnormalities, were also observed in this study, which were consistent with previous studies in patients treated with rhTPO.22,25,26 Considering that these symptoms may be related to the specific complications, such as pre-engraftment syndrome, acute GVHD, and a combination of multidrugs in transplant patients, multivariate analysis was carried out and revealed no difference between the rhTPO group and control group.
Previous clinical trials using rhTPO, romiplostim, and eltrombopag have reported a 2% to 4% incidence of thrombosis.43-45 No thromboembolic events occurred in our present study. We cannot conclude that the low incidence of thrombosis might be partly attributed to our sinusoidal obstruction syndrome or veno-occlusive disease prophylaxis protocol with low-dose heparin and PGE1. Despite a higher cumulative incidence of platelet engraftment in the rhTPO group, bleeding events were not increased in the control group with routinely prophylactic platelet transfusions. Transient anti-TPO antibodies have been detectable in a few immune thrombocytopenia cases receiving rhTPO therapy25,26 ; however, no anti-TPO antibody was detected in the present study.
It remains controversial whether TPO mimetics can potentially stimulate myeloid leukemia cell proliferation. In vitro studies revealed that TPO/MPL signaling pathways may be functional, enhancing proliferation of various types of acute myeloid leukemia and myelodysplastic syndrome cells.46-49 However, many clinical studies have confirmed the safety of TPO mimetics without increasing bone marrow malignant cell proliferation.50,51 Furthermore, some studies reported that a thrombopoietin receptor agonist may have antiproliferative activity toward leukemia cells in a leukemia cell line and a murine leukemia model.52,53 Our results showed that there was no difference in the 2-year cumulative incidence of relapse between the 2 groups. This study was conducted at a single transplant center, and the potential mechanisms underlying the results remain unclear. Thus, we cannot accurately evaluate the antitumor activity of TPO in myeloid malignancy patients.
Only in the presence of stem cells or early progenitors can a response to the TPO agent occur, but it may take from weeks to more than 1 month.54 Our previous clinical observations also revealed that the application of rhTPO from day 4 or day 8 did not promote platelet engraftment (data not shown). Therefore, we started the application of rhTPO from day 14 after UCBT in this study. Without results from a phase 1/2 study and with limited sample size, we still cannot determine the most appropriate dosage, initial application time, duration of treatment, and underlying mechanism of rhTPO in the treatment of UCBT. In addition, because of the short follow-up time, we cannot observe the long-term effects and complications in this study.
In conclusion, this study demonstrated that 2-week treatment with rhTPO beginning from day 14 after transplantation is a potentially safe, well-tolerated, and effective treatment in accelerating platelet engraftment in hematologic malignancy patients after UCBT, especially for patients who infused lower CD34+ CB cells. It is also important to evaluate the impact of rhTPO on patients receiving a higher infusion of CD34+ cells, but considering the small sample size and that they were not randomly enrolled in our study, further studies are needed. In addition, our work paves the way for further investigation of the clinical application and underlying mechanism of rhTPO and other TPO receptor agonists for the treatment of patients receiving single-unit cord blood transplantation.
Presented orally in abstract form at the 60th annual meeting of the American Society of Hematology, San Diego, CA, 1-4 December 2018.
Data may be requested from the corresponding author at xiaoyuz@ustc.edu.cn.
Acknowledgments
The authors thank American Journal Experts for assistance in editing this manuscript and all patients and their families, investigators, study coordinators, and support staff.
This work was supported by the National Natural Science Foundation of China (grant 81670165), International Cooperation Projects in Anhui Province (grant 1804b06020352), and the Fundamental Research Funds for the Central Universities (grants WK9110000001 and WK9110000060).
Authorship
Contribution: X. Zhu, B.T., L.H., and Z.S. designed the study; X. Zhu and Z.S. provided the financial support; H.L. and Z.S. provided administrative support; B.T., K.S., X. Zhang, W.Y., L.N., X.W., G.S., Y.W., J.C., H.L., and Z.S. provided study materials or patients; X. Zhu, B.T., L.H., S.C., K.S., G.S., H.L, and Z.S. collected and assembled data; Q.L., X. Zhu, B.T., L.H., and Z.S. analyzed the data; X. Zhu and B.T. wrote the manuscript; and all authors approved the final of the manuscript.
Conflict-of-interest disclosure: Q.L. received research funding from Bayer USA. The remaining authors declare no competing financial interests.
Correspondence: Xiaoyu Zhu, Department of Hematology, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui 230001, China; e-mail: xiaoyuz@ustc.edu.cn; and Zimin Sun, Department of Hematology, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui 230001, China; e-mail: zmsun@ustc.edu.cn.
References
Author notes
B.T. and L.H. contributed equally to this work.
The full-text version of this article contains a data supplement.