Key Points
Childhood- and elderly-onset sCAEBV, T- and NK-cell type, may be different disorders.
Chemotherapy is currently insufficient to resolve disease activity and eradicate infected cells of sCAEBV.
Abstract
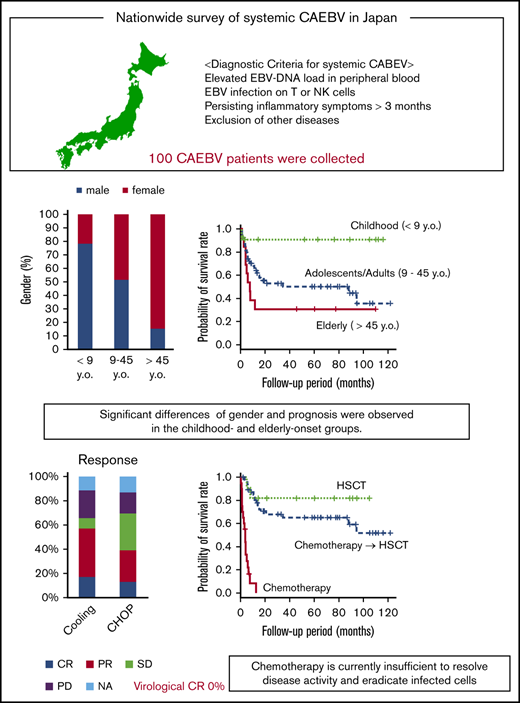
Systemic chronic active Epstein-Barr virus infection (sCAEBV) was defined as a T- or NK-cell neoplasm in the 2017 World Health Organization (WHO) classification. To clarify the clinical features of sCAEBV under this classification and review the effects of chemotherapy, we performed a nationwide survey in Japan from 2016 through 2018 of patients with sCAEBV newly diagnosed from January 2003 through March 2016. One hundred cases were evaluated. The patients were aged 1 to 78 years (median, 21) and included 53 males and 47 females. Spontaneous regression was not observed in patients with active disease. In the childhood-onset group (age, <9 years), 78% of the patients were male. In contrast, 85% of the patients in the elderly-onset group (age, >45 years) were female. The prognosis of the childhood-onset group was better than those of the adolescent/adult- and elderly-onset groups. The main chemotherapies used were a combination of cyclosporine A, steroids, and etoposide (cooling therapy) in 52 cases and cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP) in 45 cases. The rate of complete response (CR), defined as complete resolution of disease activity, was 17% for cooling therapy and 13% for CHOP. Virological CR was not observed. The 3-year overall survival rates in patients treated with chemotherapy only (n = 20), chemotherapy followed by allogeneic hematopoietic stem cell transplantation (allo-HSCT; n = 47), and allo-HSCT only (n = 12) were 0%, 65%, and 82%, respectively. Distinct characteristics were observed between childhood- and elderly-onset sCAEBV, and they appeared to be different disorders. Chemotherapy is currently insufficient to resolve disease activity and eradicate infected cells. The development of an effective treatment is urgently needed.
Introduction
Chronic active Epstein-Barr virus (EBV) infection (CAEBV) is a rare disease with persistent or recurrent inflammation accompanied by EBV infection of T or NK cells. It was originally reported as a condition with sustained inflammatory symptoms: fever, lymphadenopathy, liver dysfunction, and so-called infectious mononucleosis (IM)-like symptoms, with high titers of anti-EBV antibodies in the peripheral blood (PB).1 Later, CAEBV was reported to harbor EBV-infected clonally proliferating T or NK cells and to infiltrate the systemic organs including the PB.2-4 Some patients with CAEBV show characteristic skin lesions: hypersensitivity to mosquito bite (HMB) or hydroa vacciniforme (HV). In addition, hemophagocytic lymphohistiocytosis (HLH) or lymphoproliferative disorder (LPD)/lymphoma originating from the T- or NK-cell lineage often develop during the course of the disease.5 According to these findings, the new World Health Organization (WHO) classification, revised in 2017, defines CAEBV as an EBV+ T- or NK-cell LPD.6 The classification redefines CAEBV into 2 subtypes: systemic CAEBV (sCAEBV), accompanied by systemic inflammation, and cutaneous CAEBV of HMB or HV.
sCAEBV is a progressive disease, and to cure it, the EBV-infected, clonally proliferating T or NK cells must be eradicated. The treatment strategy currently available for eradicating EBV-infected T or NK cells in sCAEBV is allogeneic hematopoietic stem cell transplantation (allo-HSCT).5,7 Unfortunately, the presence of disease activity, defined as inflammatory symptoms, such as fever and liver dysfunction, at the beginning of the conditioning treatment of allo-HSCT, is significantly associated with poor allo-HSCT outcomes.5,8 Therefore, to improve the prognosis, patients should receive chemotherapy before allo-HSCT to resolve disease activity. However, the effects of chemotherapy on sCAEBV in a large number of patients have yet to be reported, because of the rarity of the disease.
Thus, in this study, we focused on sCAEBV and performed a nationwide survey in Japan to clarify the clinical features of sCAEBV, the current state of treatment, and the effects of the available chemotherapy regimens under the 2017 WHO classification.
Materials and methods
Study design and subjects
This was a retrospective study based on a nationwide survey conducted from 2016 through 2018 by the Japanese Study Group of CAEBV and supported by the Japanese Agency for Medical Research and Development to identify the clinical features and treatments of CAEBV.
First, we sent questionnaires to all educational hospitals certified by the Japanese Society of Hematology and those certified by the Japanese Pediatric Society. One thousand eighty-nine departments were selected, covering all 47 prefectures in Japan. We asked whether there were patients with sCAEBV that was newly diagnosed from January 2003 through March 2016. We also asked whether these patients had received chemotherapy and whether the hospital could participate in the study. We then sent a more precise survey to the institutes that responded that they could participate in the study, given that they had treated sCAEBV patients during the study period. Demographic data included sex, date of birth, date of sCAEBV diagnosis, and date of treatment initiation. A set of study sheets was completed that contained questions about clinical and laboratory findings at diagnosis, pathological findings (if applicable), types of EBV-infected lymphocytes, and treatments received and the effects. We also asked for a description of the methods used to identify the phenotypes of EBV-infected cells.
Diagnosis of sCAEBV
We reviewed the collected patients to determine whether they met the diagnostic criteria suggested by the Research Group of Measures Against Intractable Diseases of the Ministry of Health, Labor, and Welfare of Japan:
Elevated EBV-DNA load in PB (>102.5 copies/μg DNA).
EBV infection of T or NK cells in the affected tissues or PB.
Systemic inflammatory symptoms (such as fevers, lymphadenopathy, liver dysfunction, progressive skin lesions, vasculitis, and uveitis) persisting for >3 months.
Exclusion of other possible diagnoses: primary infection of EBV (infectious mononucleosis), autoimmune disease, congenital immunodeficiency, HIV, other immunodeficiencies requiring immunosuppressive therapy, or underlying diseases with potential immunosuppression.
Patients who fulfilled all criteria were diagnosed with sCAEBV. Patients with HMB only or HV-like eruptions, who exhibited hypersensitivity to sunlight by developing papulovesicular eruptions, usually on the face, without sustained systemic inflammation, were excluded from a diagnosis of sCAEBV. Patients who were pathologically diagnosed with extranodal NK/T-cell lymphoma, nasal type; aggressive NK-cell leukemia; or peripheral T-cell lymphoma, simultaneously or before diagnosis of CAEBV, were also excluded from the diagnosis of sCAEBV.
Definition of disease activity
Disease status was defined according to previous reports.5,8 Patients with active disease were defined as those with persistent findings of inflammation, as follows: fever, liver dysfunction, progressive skin lesions, vasculitis, or uveitis, accompanied by a significant increase in EBV-DNA. Liver dysfunction was defined as an increase in alanine transaminase levels to 2 times higher than the upper limit of normal. Progressive skin lesions and vasculitis were diagnosed by pathological examination. Uveitis was diagnosed by attending physicians and ophthalmologists. Status in patients without these clinical findings was defined as inactive.
Definition of response
The outcomes of the treatment were evaluated and classified as follows: partial response, resolution of a part of the findings of disease activity; complete response (CR), complete resolution of disease activity, with the EBV-DNA load in the PB remaining high (>102.5 copies/μg DNA, which is the upper limit for healthy people)10 ; virological CR (vCR), CR with a significant decrease in PB EBV-DNA load (<102.5 copies/μg DNA); progressive disease, exacerbation of active disease, or development of new findings of disease activity; and stable disease (SD), with no improvement of disease activity or novel active findings.
Statistical analysis
Comparisons among the 3 groups (children [aged <9 years], adolescents/adults, and elderly [aged >45 years]) were evaluated. The nominal variables were analyzed by Fisher’s exact test. The continuous variables were analyzed by Kruskal-Wallis test or the Mann-Whitney U test. The Kaplan-Meier method and the log-rank test were used for survival analysis. A value of P < .05 was considered statistically significant for all analyses. All statistical analyses were performed with EZR.11
Ethics statement
The current study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the ethics committee of Tokyo Medical and Dental University.
Results
Rate of patients receiving chemotherapy
A flowchart representing the study protocol is shown in Figure 1. We sent the first questionnaire to 1089 institutes and collected responses from 585 of them. Data were collected from 302 patients. Among them, 236 patients (78%) received chemotherapy and/or immunochemotherapy, and 66 patients (22%) did not receive these treatments, for reasons that are detailed in supplemental Figure 1. The most common reasons were transferring to a different hospital (41%) and SD (38%). In the internal medicine institutions, 179 of 208 (86%) patients received the treatments. The most common reasons for not receiving the treatments were transferring to a different hospital (38%) and SD (38%), whereas 17% were caused by poor general condition and death. In the pediatrics institutions, 57 of 94 (61%) patients received the treatments, which is a relatively low number. Again, the most common 2 reasons for not receiving the treatments were transfer to another hospital (43%) and SD (38%). Overall, the number of patients receiving the treatments was greater in internal medicine hospitals than in pediatric hospitals. These results suggest that there may be more adult than child patients who need treatment.
Collection of patients. (A) Flowchart of patient collection and reasons for the exclusion of some samples. (B) Distribution of age and sex at diagnosis.
Collection of patients. (A) Flowchart of patient collection and reasons for the exclusion of some samples. (B) Distribution of age and sex at diagnosis.
Patient characteristics
We sent the second questionnaire to 93 institutes, and we collected 150 patient responses from 37 institutes. Of those, we excluded 50 responses that contained insufficient information on diagnosis. The details are shown in Figure 1.
We analyzed the remaining 100 patients who met the diagnostic criteria described in “Materials and methods.” The characteristics of these patients are shown in Table 1. They were aged between 1 and 78 (median, 21) years and included 53 males and 47 females. The types of EBV-infected cells were found as follows: CD4, n = 25; CD8, n = 13; and CD56, n = 28. Three patients were γδT infected, and 2 patients were CD56− NK infected. In at least 24 patients (24%), EBV was detected in multiple lymphocyte phenotypes. Four patients were described as CD3 infected and 1 as NK infected, although phenotype details were not available. Symptoms and signs at diagnosis are presented in Table 1. The 3 major symptoms were fever, hepatosplenomegaly, and lymphadenopathy, with frequencies of 85%, 70%, and 53%, respectively. Additional symptoms, in descending order of frequency, were cardiac dysfunction, aneurysm, gastrointestinal symptoms, neurological symptoms, vasculitis diagnosed by pathology, and uveitis, at 9%, 9%, 8%, 8%, 7%, and 4%, respectively. In 24 patients, sCAEBV was accompanied by HLH. HMB was detected in 25% of the patients. No patient showed accompaniment by progressive skin lesions. In the laboratory findings, elevated alanine aminotransferase (ALT), thrombocytopenia, and elevated soluble interleukin-2 receptor (sIL-2R) were present at rates of 31%, 30%, and 27%, respectively.
CAEBV diagnostic methods
The methods of detecting EBV-infected cells were evaluated in 87 patients and were as follows: antibody-conjugated magnetic bead sorting, 61 patients (70%); flow cytometry, 18 patients (21%); and histopathology using in situ hybridization for EBV-encoded small RNA, 8 patients (9%) (supplemental Figure 2A). Seventy-nine patients (91%, 79 of 87) were diagnosed by analysis of PB mononuclear cells (PBMCs). Detection by antibody-conjugated magnetic bead sorting was performed according to the previous reports.5,12 Flow cytometric EBV-encoded small RNA in situ hybridization was performed according to the previous reports.13 Antibody-conjugated magnetic bead sorting was the most frequently used method for detecting PBMCs. The clonality of EBV-infected cells was examined in patients by Southern blot for EBV-terminal repeat14 as follows: monoclonal, 54; oligoclonal, 7; polyclonal, 4; and not described, 35 (Table 1). The specimens used to examine clonality were PBMCs, except 1, which was a histological specimen.
The diagnostic criteria for sCAEBV include detection of EBV-DNA in PB. EBV-DNA of the evaluable patients was examined as follows: in whole blood, 80; mononuclear cells, 28; plasma, 10; and not described, 1 (supplementary Figure 2). The units for EBV-DNA were not unified. Most samples (43 patients) were measured using whole blood and expressed in copies per microgram of DNA. Their median was 104.7 copies/µg DNA. In 5 patients, EBV-DNA was examined in both whole blood and plasma (supplemental Figure 2B). Among them, 1 patient was negative for EBV-DNA in the plasma.
We asked whether pathological diagnosis of CAEBV had been made. Although 14 patients had a pathological diagnosis of CAEBV, 77 did not have the diagnosis. There was no response for 9 patients. No patient was diagnosed as having T-cell lymphoma at the time of diagnosis of CAEBV.
Characteristics of clinical findings by age group
The distribution of age and sex at the time of diagnosis is shown in Figure 1B. Interestingly, we noticed that more male patients were aged <20 years, whereas more female patients were aged >45 years. Because the outcomes were shown to differ between children and adults,15,16 we divided the patients into 3 groups by age at onset to compare their clinical features and courses: childhood-onset group <9 years of age, adolescent/adult-onset group, and elderly-onset group >45 years of age. The clinical findings for each group are shown in Table 2. The number of child, adolescent/adult, and elderly patients were 23, 64, and 13, respectively. We confirmed significant sex differences in the childhood- and elderly-onset groups. In the childhood-onset group, 78% of the patients were male. In contrast, 85% of the patients in the elderly-onset group were female. No sex difference was found in the adolescent/adult-onset group.
Some findings were characteristic of each age group. The number of patients whose EBV-infected cells were CD8+ was significantly smaller in the childhood-onset group than in the other groups. No patients with aneurysm, vascular lesions, or cardiac lesions were found in the elderly-onset group. The number of platelets was lower in the elderly-onset group than in the childhood-onset group. The development of malignant lymphoma was significantly frequent in the disease course of patients in the elderly-onset group.
Outcomes
We analyzed the clinical courses and outcomes of the patients. Spontaneous remission of disease activity was not found among the 64 patients whose courses of disease activity could be evaluated.
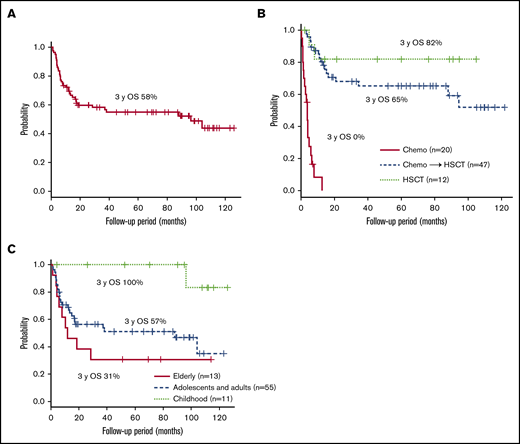
Figure 2A shows the survival of 80 patients whose clinical courses from diagnosis were available. The 3-year overall survival (OS) after diagnosis was 58% (95% confidence interval [CI]; 46%-68%). All patients were treated. The clinical courses from initiation of treatment were available in 79 patients: 12 patients were treated with allo-HSCT only, 47 patients received chemotherapy and/or immunochemotherapy followed by allo-HSCT, and 20 patients were treated with chemotherapy only. The 3-year OS after each treatment initiation is shown in Figure 2B: 82% (95% CI, 45%-95%) in patients who had allo-HSCT and 65% (95% CI, 49%-78%) in patients who had chemotherapy followed by allo-HSCT. In patients treated with chemotherapy only, the 3-year OS was, disappointingly, 0%, and all of them died of progressive sCAEBV. Among them, 18 patients did not receive allo-HSCT because the speed of progression was faster than the time taken to select appropriate donors and prepare for the transplantation. The other 2 elderly patients (75 and 78 years) were judged by their physicians to be too old for allo-HSCT. Survival in each age group is shown in Figure 2C. The 3-year OS for patients in the childhood-, adolescence/adult-, and elderly-onset groups (from the diagnosis) was 100%, 57% (95% CI, 42%-69%), and 31% (95% CI; 9.5%-55%), respectively. The prognosis of patients with childhood onset was superior to that of the other 2 groups. As shown by survival curve by age group in supplemental Figure 3, the prognosis of the patients treated with chemotherapy only was poor in all age groups. The methods of treatments and the number of patients receiving different treatments by age group are shown in supplemental Table 1. We found that patients in the elderly-onset group were treated with chemotherapy only.
The survival rates after diagnosis. (A) The survival time from diagnosis of the 80 patients whose clinical courses were available. (B-C) The survival after treatment initiation of 79 patients whose clinical courses were available. The survival rates are given for each treatment (B) and each age group (C).
The survival rates after diagnosis. (A) The survival time from diagnosis of the 80 patients whose clinical courses were available. (B-C) The survival after treatment initiation of 79 patients whose clinical courses were available. The survival rates are given for each treatment (B) and each age group (C).
Treatments used and their effects
Next, we selected 9 institutes as experienced, high-volume institutes, where >3 patients had been treated. We analyzed the treatment regimens and their effects on the patients from the selected institutes. Except for age, no significant differences in patient characteristics were found between the selected institutes and the other institutes (supplemental Table 2). Table 3 shows chemotherapy regimens that were performed in >5 patients with active disease, as defined in “Materials and methods.” Many patients were treated according to the strategies suggested by Sawada et al.17 As a first-line treatment, cooling therapy, which is a combination of steroids, cyclosporine, and etoposide, was used most frequently. The CHOP and etoposide, cytarabine, l-asparaginase, methylprednisolone, and prednisolone (ESCAP) therapies were performed most frequently as second- and third-line treatments, respectively.
Treatment effects were investigated in the 62 patients from the high-volume institutes. The effects are shown in Table 4. The CR rates for cooling therapy and CHOP were 17% and 13%, respectively. No patient achieved vCR. Adverse events are also listed in Table 4. Hematopoietic toxicities in sCAEBV tended to be more frequent than hematological malignancies.
Discussion
This study is the first large-scale report of sCAEBV performed after the publication of the new WHO classification in 2017.6 The diagnostic criteria used in this study were suggested by the Japanese study group in 20155,9 and are in line with the 2017 WHO classification. We took into consideration that our study contains many cases that were diagnosed before the classification was published.
CAEBV was originally reported as a disease of childhood.15,18 In this study, however, the number of patients >20 years of age was greater than that of patients with childhood onset, and the treatment rate was higher in adults than in children. In addition, the prognosis of adult-onset CAEBV was reported to be poorer than that of childhood-onset CAEBV,16,19 agreeing with the results of the present study. However, the reason underlying the poor outcomes of adult-onset CAEBV have not been clarified to date. Some investigators have suggested that the poor outcomes of adult-onset CAEBV are caused by lack of knowledge of the disease among internal medicine physicians, leading to delays in proper diagnosis and initiation of treatment.16,19,20 In the present study, the onset of malignant lymphoma was significantly frequent during the progress of elderly-onset disease. Oshima et al21 proposed a clinicopathological categorization of EBV-T/NK LPD, based on pathological evaluation and molecular data: category A1: polymorphic LPD, without clonal proliferation of EBV-infected cells; category A2: polymorphic LPD with clonality; category A3: monomorphic LPD with clonality. Patients with adult-onset CAEBV may have the highly malignant form, A3. In addition, we identified sex biases in the childhood- and elderly-onset groups. These findings suggest that childhood-onset sCAEBV is an independent disorder. The sex preference toward males in childhood suggests the possibility of X-linked diseases as a background. X-linked lymphoproliferative syndrome (XLP)-1 and XLP2 are inherited immunodeficiencies caused by the mutations of SH2D1A/SLAM-associated protein (SAP) and X-linked inhibitor of apoptosis protein (XIAP) genes, respectively. In these disorders, primary EBV infection induces severe EBV-associated HLH.22,23 Yang and colleagues24 analyzed the phenotype of EBV-infected cells in EBV-HLH with XIP1 and XIP2 and reported that the infected cells were mainly CD19+ B cells. However, the analysis was of a limited number of patients, and the phenotypes of EBV-infected cells in XLP has not been elucidated. XIP1, XIP2, or undetermined X-linked disorders may have contaminated childhood-onset sCAEBV data. Further study is indispensable for clarifying the immunological profile, including lymphocyte function and genetic background. The female bias of elderly-onset sCAEBV is also interesting. The clinical features of sCAEBV include chronic systemic inflammation and resemble those of autoimmune diseases, which are also sex-biased toward females. There may be common risk factors for developing both diseases. Future investigations should include detailed clinical histories, such as the presence of acquired immunodeficiency.
To diagnose CAEBV, it is necessary to determine the EBV-infected cell type. In this study, unfixed PB was used to detect the infected cells in most of the patients: 91% in all age groups. Because CAEBV does not usually manifest as solid tumors,15 it is difficult to obtain tissue specimens. However, EBV-infected T or NK cells were detected in PB in most patients with CAEBV.5 Analysis of the infected cells in PB allows for the use of minimally invasive and efficient means of acquiring specimens. In addition, this method enabled us to determine minor fractions of EBV-infected cells. EBV was detected in multiple lymphocytes phenotypes in 24% of the patients in this study. Okuno et al25 reported that EBV infection on both T and NK cells was detected in 5 of 80 patients with CAEBV. They also demonstrated some driver mutations were shared by EBV-infected cells of different lineages. These findings suggest that common progenitors of T and NK cells could be the targets of EBV infection in CAEBV. Detailed analysis of the infected cell phenotypes and their genetic features is necessary, not only to diagnose but also to clarify the mechanisms of disease development.
sCAEBV may progress rapidly or slowly, and the speed of progression varies by patient. Figure 2B shows that the 3-year OS of the patients treated with chemotherapy followed by allo-HSCT was lower than that of the patients treated by allo-HSCT only. However, the former may have included severe cases that required chemotherapy before transplantation. On the contrary, we suspect that the patients treated only with allo-HSCT included a large number of nonsevere cases in which the symptoms progressed slowly. Thus, we cannot conclude that treatment only with allo-HSCT is superior to chemotherapy followed by allo-HSCT.
The results of this study differ in some respects from those of previous studies. Kawamoto et al19 reported different clinical features between pediatric (age, <15 years) and adult-onset CAEBV, including significantly decreased incidence of fever but greater frequency of skin lesions in adult-onset cases. However, in the present study, fever was the most frequent symptom in every age group, with no apparent difference between the groups. In addition, no patients had progressive skin lesions in either childhood- or adult-onset cases. In fact, the number of patients in whom diagnosis of CAEBV was made by histolopathological examination in the present study was 14 (15%). In the report of Kawamoto et al,19 adult-onset CAEBV was diagnosed by histopathology, and pediatric-onset case information was obtained from the 2012 report of Kimura et al.5 In that report, unfixed PB was used to determine the phenotypes of EBV infection, as in most of the patients in the present study. Therefore, the differences between our results and those of Kawamoto et al19 may have been caused by patient selection bias, especially with respect to adult cases. Pathophysiological differences can exist among CAEBV patients with and without lesions available for biopsy.
The measurement unit of EBV-DNA was not determined in the present study. In addition, the measurement had not been standardized before. In 2016, the WHO International Standard for EBV was released that recommended quantitative polymerase chain reaction for measuring viral load.26 Our research group measured the EBV-DNA load in a standardized unit of measurement, using this reference material, and found that the load of CAEBV was relatively high among the patients with EBV-related diseases.27 In the future, we must determine the association of EBV-DNA load and CAEBV clinical features in a study based on a larger number of cases.
This study has limitations. First, the number of patients was relatively small. As shown in Figure 1A, the number of the patients collected by the first questionnaire was 302, and the number collected by the second one was 150. Participation in the study was not compulsory, and the response rate to the second questionnaire was only 50%. Second, there may have been a patient-selection bias. Among the 302 patients identified by the first questionnaire, 78% received chemotherapy and/or immunochemotherapy. All 80 patients identified by second questionnaire, whose clinical courses from diagnosis were available, received these treatments. These findings suggest that the patients analyzed in the present study may be biased toward having more severe conditions. Third is the low ratio of pathological diagnosis. There were only 14 patients who were pathologically diagnosed as having CAEBV, whereas 77 patients were not. We assume that it was difficult to diagnose the disease morphologically in some patients or to obtain specimens from the affected organs, such as the liver or spleen. CAEBV does not usually manifest as solid tumors. In sCAEBV, local lesions do not usually develop. In addition, many patients have thrombocytopenia and vascular lesions. Therefore, performing a biopsy is usually challenging. On the other hand, as mentioned earlier, EBV-infected cells can be analyzed in the PB. We need more discussion on pathological diagnosis and its significance. Finally, this was a retrospective study. To confirm the results of the present study and clarify prognostic factors, a prospective analysis based on a registry for this disease is indispensable. The Research Group of Measures against Intractable Diseases of the Ministry of Health, Labor, and Welfare of Japan is planning to establish such a registry; thus, an unbiased, large-scale analysis will be available.
In the present study, no treatment regimen brought about vCR. Furthermore, chemotherapy was insufficient to resolve disease activity. According to previous reports, patients with active disease at allo-HSCT had poorer prognoses than did those without active disease.5,8 It is necessary to control the disease activity before allo-HSCT. The number of patients with CAEBV is expected to increase, given the new description of CAEBV in the WHO classification, and thus, development of effective drug therapies is an urgent need. We recently found and reported that NF-κB and STAT3 were constitutively activated in EBV-infected cells in CAEBV.12,28 STAT3 especially mediates the molecular signaling of the receptors of the multiple inflammatory cytokines. Our results demonstrated that JAK inhibitors, including ruxolitinib, suppressed not only STAT3 activity, but also the production of the cytokines.12 STAT3-mediating pathways can be attractive therapeutic targets, not only for resolution of inflammation but also for eradication of EBV-infected cells.
Original data may be requested by e-mail to the corresponding author.
Acknowledgments
The authors thank Ayako Komoto, an assistant supported by the funding from the Japanese Agency for Medical Research and Development (AMED), for excellent editorial support to the authors during the preparation of the manuscript, and all the doctors involved in the questionnaire survey for their contributions.
This work was supported by grants from the Practical Research Project for Rare/Intractable Diseases (18ek0109334h0001, 19ek0109334h0002, and 20ek0109334h0003) from AMED.
Authorship
Contribution: I.Y., C.S., K.-I.I., T.K., and A.A. designed the research, performed the survey, and analyzed the data; A.S., Y.I., N.F., A.H., Y.T., M.M., T.E., S.K., S.O., and M.I. performed the survey; M.Y., O.M., H.K., and S.F. analyzed the data; and all authors contributed to the modification of the draft and approved the final submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ayako Arai, St. Marianna University School of Medicine, 2-16-1 Sugao, Miyamae-ku, Kawasaki, Kanagawa, Japan; e-mail: ara.hema@marianna-u.ac.jp.
References
Author notes
The full-text version of this article contains a data supplement.