TO THE EDITOR:
We previously reported the results of the INO107 study,1 a phase 3 randomized, open-label, multicenter study comparing inolimomab (an anti-CD25 monoclonal antibody blocking interleukin-2 receptor α) vs antithymocyte globulin (ATG) in adult patients with steroid-refractory acute graft-versus-host disease (SR-aGVHD). The study was conducted between April 2009 and February 2015, and a total of 100 patients were randomly assigned: 49 patients in the inolimomab arm and 51 patients in the ATG arm. The primary end point, therapy success, was defined as overall survival (OS) at 1 year without changing the therapy allocated at baseline. The primary objective was not achieved in this trial: therapy success was reached by 14 patients (28.5%) in the inolimomab arm and 11 patients (21.5%) in the ATG arm (adjusted hazard ratio [HR], 0.722; P = .188). Analysis of OS alone showed an interesting trend in favor of inolimomab (adjusted HR, 0.628; P = .11).
The protocol was approved by institutional ethics review boards, and the study was performed according to the Helsinki guidelines. This trial was registered as European Union Drug Regulating Authorities Clinical Trials (EudraCT) 2007-005009-24.
At the end of the 1-year follow-up period of the INO107 study, 43 patients were alive (23 [47%] in the inolimomab arm and 20 [40%] in the ATG arm). Centers were contacted in 2018 to collect long-term follow-up (LTFU) data regarding survival or death (after 1 year), including date and causes of death and the occurrence or not of chronic GVHD (cGVHD). Here we report the analysis of these LTFU data in the intent-to-treat population (n = 100) with a cutoff date of 16 May 2018.
For OS end points, hypothesis testing was performed by using an adjusted Cox proportional hazard regression model with a 2-sided 0.05 level of significance. The treatment effect was adjusted for the following prespecified covariates: hematopoietic stem cell transplantation remission, sex mismatch, family link, and skin, liver, or gut involvement. Patients who had not experienced an event at the cutoff date were right censored on the date of their last contact.
To assess the robustness of the results obtained in the OS analysis, the Cox regression model was assessed by using the Schoenfeld and martingale residuals. cGVHD was analyzed by using a Cox proportional hazard model with the Fine and Gray competing risk method, including the covariates used for the primary end point. Death was the competing risk. The significance level was set at 5% (2-sided).
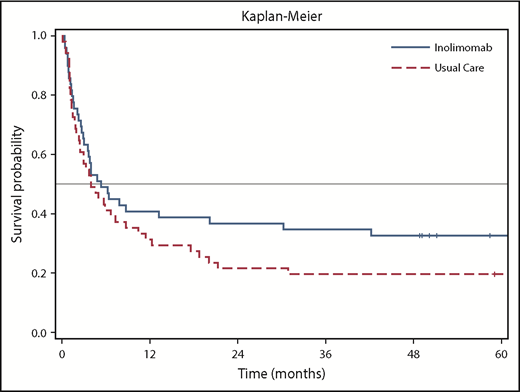
The follow-up duration for the 43 patients varied up to 104 months (8.7 years), with a median of 58.4 months. Fifteen patients (30.6%) were alive in the inolimomab arm and 10 (19.6%) were alive in the ATG arm at the end of follow-up (Figure 1). The adjusted HR was 0.572 (95% confidence interval, 0.346-0.947; 2-sided P = .030). This represents an absolute difference of 11% and a relative difference of 56% in OS in favor of inolimomab. The difference in OS reached statistical significance (P = .030) with a relative reduction of risk of 43%. In addition, the HR of the LTFU for OS was consistent with that of the 1-year analysis (HR, 0.628). Table 1 provides the causes of death from the date of random assignment until the end of follow-up.
There are 2 times more deaths related to infection and cGVHD in patients treated with ATG than in those treated with inolimomab. Fourteen percent of patients died of recurrence of initial disease in the inolimomab arm compared with none in the ATG arm, which raises a question about the graft-versus-leukemia effect of inolimomab, considering its activity in aGVHD. Indeed, mortality related to aGVHD increased by 40% in the ATG group.
The analysis of cGVHD showed no difference between the 2 groups (HR, 0.96; 95% confidence interval, 0.45-2.02; P = 0.91). The proportion of cGVHD was similar in both groups. Cumulative incidence of cGVHD was 31% in the inolimomab group and 34% in ATG group.
Despite prevention strategies, hematopoietic stem cell transplantation is still associated with a high incidence of aGVHD.2,3 The consensus first-line treatment of aGVHD is high dose steroids.4 However, response rates are still only approximately 50% and around half the patients develop SR-aGVHD. To date, no proven second-line therapy for SR-aGVHD has been uniformly adopted or approved. Currently, only 2 randomized studies of SR-aGVHD have been published.1,5
The INO107 study was a prospective, randomized study comparing ATG vs inolimomab in patients with SR-aGVHD. This study is the first reporting the long-term follow-up (up to 104 months) of a randomized trial in this setting. Although the primary objective of the initial study was not reached after 1 year, a statistically significant difference in OS was observed in favor of inolimomab from the LFTU analysis. Furthermore, the survival curves for both groups show that the effect observed at the end of the INO107 study after a 1-year follow-up was sustained over the long term. Although this post hoc LFTU analysis was not prespecified in the study protocol, the results are encouraging and of scientific value from a clinical perspective.
There were 2 issues in this trial that influenced the decision to use ATG. First, at the time of study initiation, no drug was approved for SR-aGVHD, and standard of care was based on immunosuppressive drugs such as ATG used off-label. Second, the patients with SR-aGVHD who were treated with ATG had one of the longest lasting follow-up periods of any study up to that time, and they served as the control group of the only available randomized study in this setting.5 Although extrapolating results from this study toward another potential alternative drug is difficult, no drug has definitively proven superiority over any other drugs, so far.
This analysis may have another possible limitation linked to a bias after the initial 1-year period that could have had an impact on the results. In particular, we do not have information on therapeutic interventions after 1 year of follow-up and any potential imbalance between treatment groups in this regard. However, the analysis of OS is considered the most robust efficacy criterion for SR-aGVHD and is very unlikely to be associated with any bias. The curves for OS follow the same pattern and are in alignment with the 1-year follow-up results.1 The initial protocol was based on the hypothesis of HR being 0.75 in favor of inolimomab. This difference was already achieved at the 1-year follow-up but was not statistically significant, which highlights the issue of the lack of statistical power of the INO107 study as reported in the initial publication.1 The LTFU with accumulation of additional events that we describe is an indirect means of increasing the power of the initial study and results in statistically significant findings.
Overall, this LTFU analysis shows a clinical benefit in favor of inolimomab (30% survival) compared with ATG (19.6% survival) and suggests that inolimomab may be a suitable therapeutic alternative in patients with grade II to IV SR-aGVHD. In the meantime, the potential benefits of inolimomab vs products other than ATG currently used in the management of SR-aGVHD still need to be evaluated. The results could be the basis for further clinical investigations. In addition, the absolute numbers for OS still need to be improved, and they reinforce that we still need better therapy in the setting of SR-aGVHD.
Contribution: J.-P.V. and G.S. designed the study; G.S., N.M., I.Y.-A., J.-O.B., S.F., K.B., F.S., M.M., P.L., and J.-P.V. provided the patients; C.M. collected data and supervised the analysis; D.L. analyzed and interpreted the data; E.G. performed statistical analysis; G.S. wrote the manuscript; and all other authors critically read and approved the manuscript.
Conflict-of-interest disclosure: G.S., M.M., D.L., and E.G. are consultants and C.M. is an employee of Elsalys Biotech. The remaining authors declare no competing financial interests.
Correspondence: Gérard Socié, Hematology Transplantation, Assistance Publique–Hôpitaux de Paris, Hôpital Saint-Louis, 1 Ave Claude Vellefaux, 75475 Paris Cedex 10, France; e-mail: gerard.socie@aphp.fr.