Key Points
P falciparum growth is not inhibited in either cord or heterozygote hereditary persistence of fetal hemoglobin erythrocytes.
P falciparum growth in fetal hemoglobin erythrocytes is oxygen independent.
Introduction
The risk of malarial disease is significantly reduced in the first few months of life and increases steadily therafter.1 This relative protection has been generally attributed to maternal antibodies transmitted to the infant in utero or via breast milk2,3 and, by some, to the presence of fetal hemoglobin (HbF).4 It had also long been accepted that HbF impairs Plasmodium falciparum growth, a phenomenon first observed in cord blood erythrocytes in vitro.5,6 These studies were only performed on a small number of samples and have not been reproduced.7,8 A recent clinical trial suggests that increased HbF secondary to hydroxyurea use may be protective against malaria.9 However, HbF has been shown to increase clinical complications of severe malaria in adult patients with sickle cell anemia.10 Therefore, the effect of HbF on malaria growth needs to be clarified.
Methods
We used sensitive tools to investigate the effect of HbF on P falciparum growth in red blood cells (RBCs) in both high and low oxygen concentrations. We performed a series of experiments detailing P falciparum parasite proliferation within cord, heterozygous hereditary persistence of HbF (HPFH [hemoglobin A/HPFH (A/HPFH)]), and adult normal blood erythrocytes. In most experiments, P falciparum 3D7 IG06 parasites, with an intraerythrocytic developmental cycle (IDC) time of ∼40 hours, were used. D10-PfCDPK, parasites that can be induced to arrest just prior to egress from erythrocytes,11 were used to assess terminal merozoite numbers. The Drager Pac III gas monitor detector sensor was used to verify incubator high and low oxygen concentrations. For each experiment, synchronized schizonts obtained via MACS magnet purification were used to invade target RBCs at 1% hematocrit. Parasite growth in target erythrocytes was quantified for 1 to 2 life cycles by light microscopy, using May-Grünwald-Giemsa–stained cytospins, and by flow cytometry using SYBR Green nucleic acid dye to detect parasite DNA.12
Results and discussion
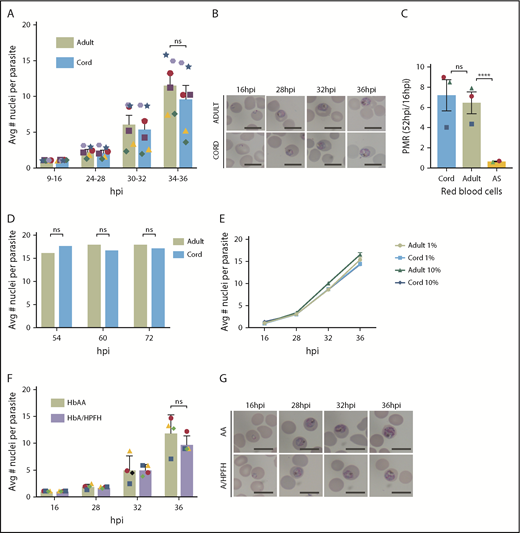
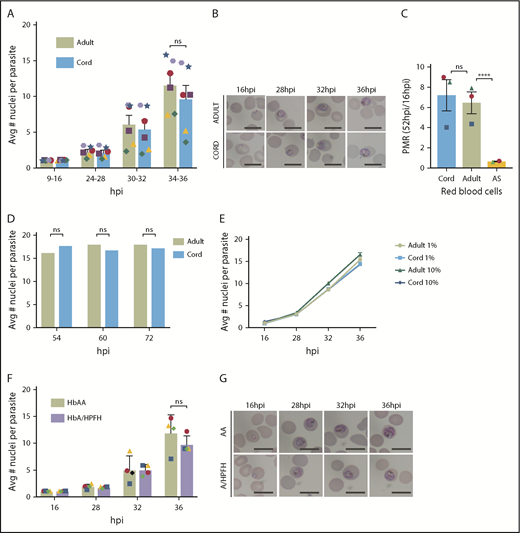
Previous in vitro studies of P falciparum growth in high HbF erythrocytes have been limited by low sample numbers, lack of single-cell approaches such as flow cytometry to count parasites, absence of magnetic-activated cell sorting synchronization followed by timed sampling throughout the IDC, and assessment in only high oxygen concentration.5,6 The present study involves fine temporal mapping of the intraerythrocytic growth of P falciparum in cord and A/HPFH RBCs. In contrast to previous reports, we find that in vitro growth and proliferation in cord blood erythrocytes or A/HPFH erythrocytes is not significantly delayed compared with growth in adult hemoglobin AA (HbAA) RBCs. In Figure 1A, we demonstrate similar growth of P falciparum 3D7 IG06 parasites in both adult and cord blood erythrocytes, which typically contain ∼80% to 95% HbF. In addition to no significant difference in the average number of nuclei per parasite in both normal adult and cord RBCs, there is no difference in morphology at any of the selected time points of the cell cycle (Figure 1B). Using the parasite multiplication rate (PMR) as a measure of 1 complete IDC, from rings at 16 hours postinfection (hpi) to rings at 52 hpi, in 1% oxygen, we show no difference in proliferation between normal adult and cord blood RBCs. This is compared with the significant depression of proliferation in sickle cell trait (HbAS) erythrocytes in low oxygen, as we previously reported12 (Figure 1C). Moreover, no significant differences in the mean number of parasite nuclei in adult and cord blood were observed at any of the time points measured, suggesting similar growth and terminal development within the different erythrocytes (Figure 1D). Importantly, the growth in adult and cord blood is oxygen independent, with no significant differences in growth curves in adult and cord blood RBCs at 1% and 10% O2 (Figure 1E). Lastly, in A/HPFH erythrocytes, there is again no significant difference in peak DNA replication through the IDC or in morphology (Figure 1F-G).
P falciparum growth and proliferation are not inhibited in HbF-containing erythrocytes. (A) Flow cytometry analysis of average number of nuclei per parasite in synchronized, RNase-treated, SYBR Green–stained P falciparum 3D7 IG06 parasites grown in normal adult (AA) and cord blood erythrocytes at 9 to 16, 24 to 28, 30 to 32, and 34 to 36 hpi in 1% O2 demonstrating no significant growth differences using paired analysis in each time point of the cell cycle; N = 6 experiments. (B) Representative Pf3D7 IG06 parasites in thin smears of adult (top) and cord (bottom) blood erythrocytes at 16, 28, 32, and 36 hpi. May-Grünwald-Giemsa stain; scale bars, 10 μm. (C) Parasite multiplication rate (PMR) of Pf3D7 IG06 parasites in cord blood and normal adult blood erythrocytes are similar in 1% O2. PMR in sickle cell trait (AS) RBCs is significantly reduced in low O2; N = 2-3 experiments. (D) Flow cytometry analysis of average number of nuclei per parasite in synchronized, RNase-treated, SYBR Green–stained P falciparum CDPK5 D10 parasites off shield demonstrating equal terminal merozoites in both normal adult and cord blood erythrocytes; N = 2000 infected RBCs counted. (E) Overlap of flow cytometry–based growth curves of average number of nuclei per parasite in synchronized, RNase-treated, SYBR Green–stained Pf3D7 IG06 parasites grown in normal adult (AA) and cord blood erythrocytes at 16, 28, 32, and 36 hpi in 1% and 10% oxygen; N = 3 experiments. (F) Flow cytometry analysis of average number of nuclei per parasite in synchronized, RNase-treated, SYBR Green–stained Pf3D7 IG06 parasites grown in normal adult (AA) and A/HPFH blood erythrocytes at 16, 28, 32, and 36 hpi demonstrating no significant growth difference. (G) Representative Pf3D7 IG06 parasites in thin smears of adult (top) and A/HPFH (bottom) blood erythrocytes at 16, 28, 32, and 36 hpi also demonstrating no difference in morphology. May-Grünwald-Giemsa stain; scale bars, 10 μm. Each colored icon signifies an individual experiment. Error bars are standard error of the mean; ****P = .0001. Avg, average; ns, not significant.
P falciparum growth and proliferation are not inhibited in HbF-containing erythrocytes. (A) Flow cytometry analysis of average number of nuclei per parasite in synchronized, RNase-treated, SYBR Green–stained P falciparum 3D7 IG06 parasites grown in normal adult (AA) and cord blood erythrocytes at 9 to 16, 24 to 28, 30 to 32, and 34 to 36 hpi in 1% O2 demonstrating no significant growth differences using paired analysis in each time point of the cell cycle; N = 6 experiments. (B) Representative Pf3D7 IG06 parasites in thin smears of adult (top) and cord (bottom) blood erythrocytes at 16, 28, 32, and 36 hpi. May-Grünwald-Giemsa stain; scale bars, 10 μm. (C) Parasite multiplication rate (PMR) of Pf3D7 IG06 parasites in cord blood and normal adult blood erythrocytes are similar in 1% O2. PMR in sickle cell trait (AS) RBCs is significantly reduced in low O2; N = 2-3 experiments. (D) Flow cytometry analysis of average number of nuclei per parasite in synchronized, RNase-treated, SYBR Green–stained P falciparum CDPK5 D10 parasites off shield demonstrating equal terminal merozoites in both normal adult and cord blood erythrocytes; N = 2000 infected RBCs counted. (E) Overlap of flow cytometry–based growth curves of average number of nuclei per parasite in synchronized, RNase-treated, SYBR Green–stained Pf3D7 IG06 parasites grown in normal adult (AA) and cord blood erythrocytes at 16, 28, 32, and 36 hpi in 1% and 10% oxygen; N = 3 experiments. (F) Flow cytometry analysis of average number of nuclei per parasite in synchronized, RNase-treated, SYBR Green–stained Pf3D7 IG06 parasites grown in normal adult (AA) and A/HPFH blood erythrocytes at 16, 28, 32, and 36 hpi demonstrating no significant growth difference. (G) Representative Pf3D7 IG06 parasites in thin smears of adult (top) and A/HPFH (bottom) blood erythrocytes at 16, 28, 32, and 36 hpi also demonstrating no difference in morphology. May-Grünwald-Giemsa stain; scale bars, 10 μm. Each colored icon signifies an individual experiment. Error bars are standard error of the mean; ****P = .0001. Avg, average; ns, not significant.
Whether HbF provides protection from P falciparum malaria infections (and if so, how) is an important controversy to resolve. Despite previous accounts suggesting significant growth impairment in cord blood RBCs, we found that growth in cord blood erythrocytes was not significantly delayed compared with growth in adult (HbAA) erythrocytes, similar to results observed by Amaratunga et al8 and Sauerzopf et al.7 The first studies of parasite growth in HbF-containing RBCs were performed by Pasvol and Weatherall et al in 19765 and 1977.6 In those well-designed studies, several different types of erythrocytes rich in HbF were used, taking into consideration the age of the erythrocyte and the distribution of HbF. However, clinical isolates and a complete IDC and subsequent growth were not interrogated, precluding a full analysis of proliferation. The studies were halted prematurely as evidenced by the fact that schizonts, even in hemoglobin A (HbA)–containing red cells (eg, in adult HbAA), were very rare (13% schizonts and 83% trophozoites).6 In our studies, we considered growth and proliferation through the entire IDC, and terminal merozoite formation was equal in both cord and adult (HbAA) RBCs (Figure 1). There is a readily apparent variation in growth between the unique blood samples used for different experiments (illustrated using varying colored icons). We speculate that this is less likely related to differences between the RBCs, and more likely related to the age of the schizont parasites at the time of initiation of the studies, given the similar growth profiles of matched HbF-containing and normal adult RBCs.
There are several differences between cord and normal (HbAA) adult RBCs including cellular metabolism, mean corpuscular volume, and mean hemoglobin content per cell. Cord RBCs have higher oxygen-binding affinity due to HbF’s low affinity for 2,3-bisphosphoglyceric acid. Given the profound effect of low oxygen on HbAS proliferation, we found it necessary to assess growth in both low and high oxygen. Previous experiments limited their evaluation to high oxygen.5-8 We found no difference in P falciparum growth in cord blood erythrocytes in 1% and 10% oxygen. To further isolate the effect of HbF, we examined African A/HPFH erythrocytes, which offer a better comparison with normal adult RBCs because the only difference between these African A/HPFH RBCs and normal adult RBCs is the level of HbF. In addition, unlike the HPFH homozygous RBCs used in the work by Amaratunga et al,8 these RBCs have a value of HbF comparable to sickle cell disease patients treated with hydroxyurea. In such African A/HPFH erythrocytes, with HbF of 30%, no significant parasite growth delay was observed.
Direct measurement of DNA replication with multiple time points within the IDC demonstrates that HbF does not significantly affect parasite growth in erythrocytes. These results support the recent work of Sauerzopf et al7 that measured proliferation in multiple cord blood samples. Future studies should also consider the use of multiple parasite strains and clinical isolates. An effect on growth was also not seen in low oxygen, contrary to that observed in HbAS erythrocytes.12 Lastly, parasites in African A/HPFH RBCs with ∼30% HbF, similar to the HbF values in the RBCs of those with sickle cell disease treated with hydroxyurea in Tshilolo et al,9 also showed no reduction in parasite growth compared with parasites in adult (HbAA) erythrocytes. These data support the conclusion that HbF does not negatively affect malaria growth. If HbF is protective of malaria infection, it may rely on other protective mechanisms such as cytoadherence or enhancement of antibody influence. Growth inhibition should not be considered a factor.
Acknowledgments
The authors express thanks to the women who generously volunteered to donate cord blood for this study and the individuals with A/HPFH who donated their peripheral blood. The authors thank Carlo Brugnara, H. Franklin Bunn, Daniel Goldberg, Jane Hankins, and David A. Williams for their contributions to this project. David G. Nathan was instrumental in forming the initial study aims and for that the authors are very grateful.
This work was supported by grants from the Harvard Catalyst Program for Faculty Development and Diversity Inclusion Faculty Fellowship, the American Society of Hematology/Robert Wood Johnson Foundation Harold Amos Medical Faculty Development Award, the Eleanor and Miles Shore Fellowship Program Dana-Farber Cancer Institute Fellowship, and the National Institutes of Health, National Heart, Lung, and Blood Institute (1R01 HL139337).
Authorship
Contribution: N.M.A. proposed the study and performed flow cytometry analyses; N.M.A. and M.T.D. conceived the experiments and wrote the manuscript with significant contributions from N.P.; and N.M.A. and N.P. performed the parasite growth and proliferation assays.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Manoj T. Duraisingh, Department of Immunology and Infectious Diseases, Harvard University, 651 Huntington Ave, FXB Building, Room 205, Boston, MA 02115; e-mail: mduraisi@hsph.harvard.edu.