Background
Nicaragua is a low-income country located in Central America. The country has a multiethnic population, with Mestizos, American Indian, and black being the most predominant ethnic groups. Nicaragua lacks a national newborn screening program and facilities dedicated to the study of hemoglobinopathies. Sickle cell disease (SCD) is diagnosed on the basis of the patient’s clinical history and routine hematologic analysis. The prevalence of this pathology is unknown.
Objectives
To build a specialized laboratory for hemoglobin research.
To confirm the clinical diagnosis of SCD in patients.
To determine the prevalence of SCD, sickle cell trait (SCT), and other hemoglobin variants.
Hypothesis
Accurate diagnostic techniques must be implemented to correctly detect and classify hemoglobinopathies.
Low prevalence of SCD can be detected by using cellulose acetate electrophoresis (CAE).
Methods
Study design
A cross-sectional study was conducted in 2 different populations. The first group consisted of clinically diagnosed SCD patients, and the second group consisted of healthy individuals from several parts of the country. Peripheral blood was collected and taken to the newly established Molecular Biology Laboratory at the National Autonomous University of Nicaragua for testing.
Laboratory analysis
The presence of abnormal hemoglobin was confirmed by CAE (Figure 1). If necessary, high-performance liquid chromatography and DNA sequencing were used to confirm hemoglobin variants.
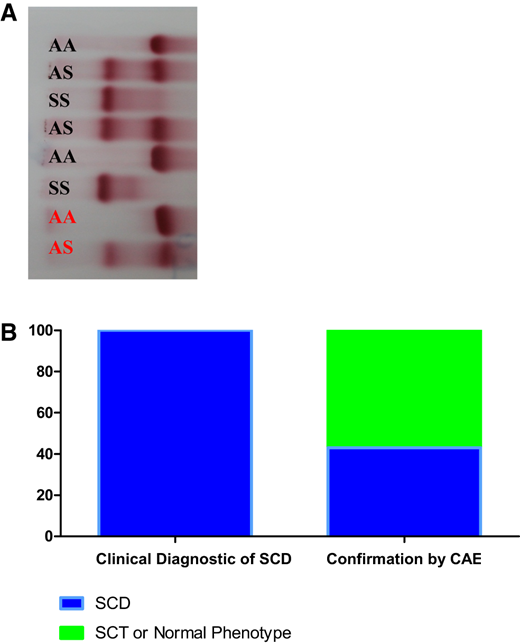
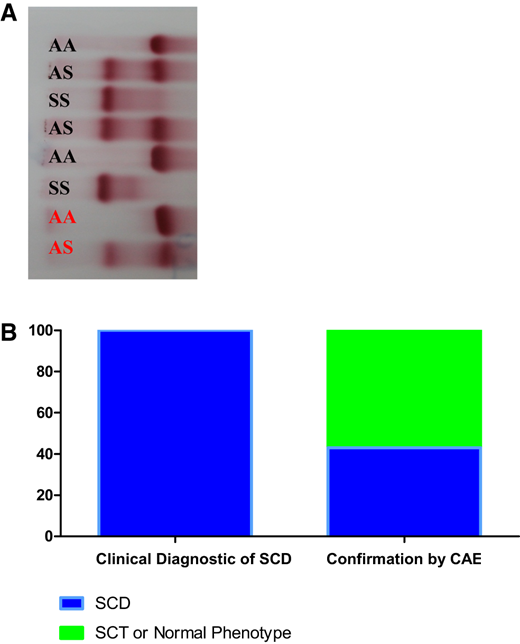
Confirmation of clinical diagnosis of SCD. (a) CAE of hemolysates. Patients are shown in black and hemolysate controls are shown in red. (b) Comparative results between clinical diagnosis of SCD and confirmation of SCD, SCT, or normal phenotype by CAE.
Confirmation of clinical diagnosis of SCD. (a) CAE of hemolysates. Patients are shown in black and hemolysate controls are shown in red. (b) Comparative results between clinical diagnosis of SCD and confirmation of SCD, SCT, or normal phenotype by CAE.
Capacity building
A new research laboratory for studying hemoglobin was created in the Polytechnical Institute of Health at the National Autonomous University of Nicaragua, with collaboration by the German Academic Exchange Program.
One laboratory technician and 10 clinical bioanalysis students were trained to perform CAE.
All CAE tests were performed on a daily basis in a local facility.
CAE results were interpreted by a specialist.
Rare hemoglobin variants were sent to collaborators in Costa Rica and the United States for confirmation.
Results
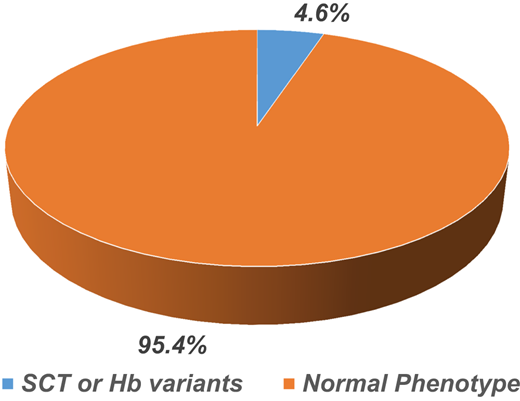
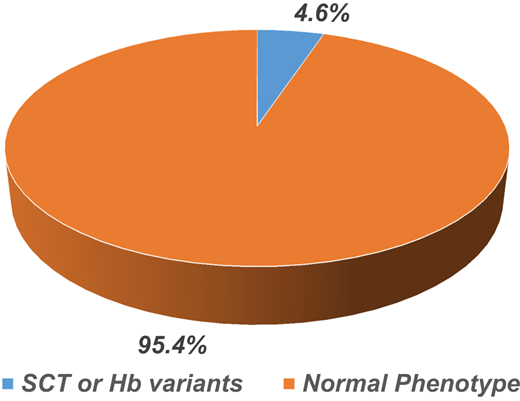
In the first group, 150 blood samples from patients with a clinical diagnosis of SCD were analyzed over a 2-year-long study period. Sixty-five patients (43%) with the hemoglobin phenotype of SCD were confirmed. However, 85 patients (57%) had an SCT or normal phenotype. In the second group, 450 blood samples from healthy individuals were screened, 21 (4.6%) were positive for SCT, and the other 429 (95.4%) were normal (Figure 2).
Conclusion
Developing a hemoglobin research laboratory improved the accuracy of diagnosing SCD and other hemoglobin disorders. Screening healthy individuals provided valuable information regarding the prevalence of hemoglobinopathies which could be used to design prevention programs.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Allan Pernudy-Ubau, Molecular Biology Laboratory “M.A. Elmer Cisneros in Memoriam,” Polytechnic Institute of Health, National Autonomous University of Nicaragua, Managua, Nicaragua; e-mail: pernudi@gmail.com.