Background
Sri Lanka, an island in the Indian Ocean with an area of 62 705 square kilometers, has a population of 21 million people. It is divided into 9 provinces, 25 districts, and 324 divisional secretary areas (Figure 1). In Sri Lanka, the public sector provides universal free health coverage. The Department of Health and the provincial health sector cover the whole range of curative and preventive services. Life expectancy at birth (in 2016) was 72 years for males and 78 years for females. Total expenditure on health per capita is Intl$369 (3.5% of gross domestic product). The ratio of physicians to people is 1.04 per 1000 (70% of physicians are employed in the public sector).
The National Cancer Institute (NCI), the only cancer hospital in Sri Lanka, was established in 1958 by government health services. The NCI is located in the greater Colombo area and provides cancer services to people in the greater Colombo area as well as to the patients referred from all other peripheral oncology units in the country. About 13 000 new patients with cancer are registered at NCI annually (Figure 2). NCI has been recognized as a principal curative center, postgraduate training unit, and research institution of the field of oncology. The absence of hematopoietic stem cell transplantation (HSCT) at NCI is a major deficiency, because most patients who need HSCTs cannot afford to go abroad for them.
In 2014, a London-based tea company, Ahmad Tea, donated funding for the new Razavi Medical Complex (valued at SLR1.2 billion [SLR, Sri Lankan rupee]) to NCI where a bone marrow transplantation (BMT) unit with 2 rooms was developed with provisions to expand to 6 more rooms.
Hematologic disease burden in Sri Lanka
About 1100 new hematologic malignancies are diagnosed each year in Sri Lanka of which 25% are plasma cell disorders. Seventy percent of hematologic malignancies diagnosed in Sri Lanka are treated at NCI.
We describe the establishment of an HSCT center at the new NCI Razavi Medical Complex (Figure 3). In June 2014, John Moore and David Ma of St Vincent’s Hospital (SVH), Sydney, Australia, were approached by the Australian Sri Lanka Society, which asked for help and expertise in the field of HSCTs. A year later, with support from the government of Sri Lanka, the Sri Lankan High Commissioner in Australia, and an education grant from the Australian Federal Government and the Australian Sri Lanka Association, a 16-member team from NCI consisting of an oncologist, a hematologist, a transfusion physician, a microbiologist, 4 medical officers, 2 laboratory technicians, a pharmacist, and 5 nurses received initial intensive education and training from the team at SVH (Figures 2-4).
After returning from SVH, the NCI team continued its training and helped prepare the facilities. Through regular videoconferences and e-learning programs, the team in Sydney offered continuous mentoring and helped to design the transplantation ward and stem cell transplantation facilities. In late 2016, the team performed the first ever transplant under the guidance of a 5-member team from SVH (Figures 5-8). During the first year, 20 autologous transplantations were performed.
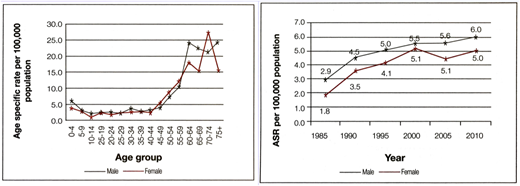
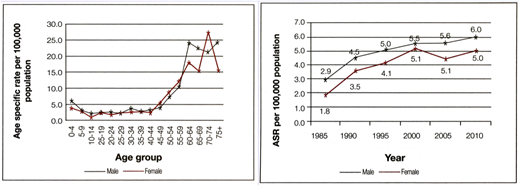
Cancer incidence data from Sri Lanka's National Cancer Control Programme. ASR, age-specific rate.
Cancer incidence data from Sri Lanka's National Cancer Control Programme. ASR, age-specific rate.
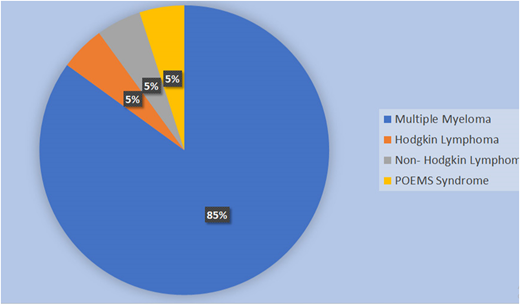
Disease percentage among patients at National Cancer Institute, Sri Lanka, during the first year.
Disease percentage among patients at National Cancer Institute, Sri Lanka, during the first year.
Preparation
Pretransplant: NCI constructed an HSCT facility at the newly built Razavi Medical Complex and upgraded the pathology facilities. The National Blood Transfusion Service (NBTS) in Sri Lanka undertook apheresis, stem cell processing, and cryopreservation.
Transplant eligibility: Patients who had multiple myeloma (MM) and were in complete remission or partial remission, had relapsed non-Hodgkin lymphoma (NHL) or Hodgkin lymphoma (HL), and were younger than age 65 years with good performance status were selected and included.
Autologous HSCs were mobilized with cyclophosphamide (2 g/m2) and granulocyte colony-stimulating factor (10 μg/kg/day for 8-9 days). HSC products were cryopreserved, and samples that had been thawed were checked for viability and contamination before product infusion.
Melphalan (200 mg/m2) was the conditioning regimen for patients with MM, and carmustine, etoposide, cytarabine, and melphalan (BEAM) was the regimen used for those with HL and NHL. Patients received intensive monitoring around the time of the transplant. Immediate commencement of antibiotics, fluid management, and blood component transfusions were implemented per protocols. Engrafted patients were discharged and were managed regularly at the clinic.
Results
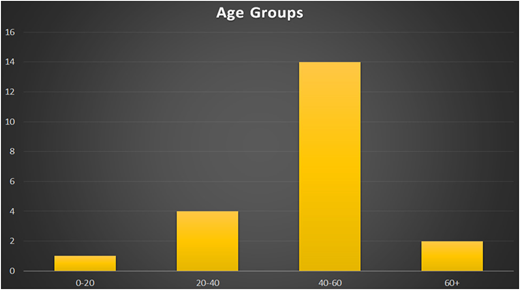
Twenty autologous transplantations were performed in the first year. The mean age for patients was 47 years (range, 17-62 years), and the male:female ratio was 3:2 (Figure 9). There were 17 patients with MM and 1 each with NHL, HL, and POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes) (Figure 10).
The median for collected CD34 stem cells was 12.72 × 106/kg (range, 3 to 31.7 × 106/kg). Sixty percent of the patients needed single apheresis. Median infused cell dose was 4.07 × 106/kg (range, 2 to 7.4 × 106/kg).
Median engraftment day was day 13 (range, days 11-19). Median hospitalization stay was 16 days (range, 14-20 days). All patients developed febrile neutropenia (7 had positive cultures). All patients developed grade 3 thrombocytopenia and needed platelet transfusions (median platelet packs, 12). No patients experienced transplant-related mortality, and acceptable morbidity was achieved.
At the median follow-up of 47 weeks (to May 31, 2018), the overall survival was 100%. Eleven patients with MM (65%) were in remission.
Conclusion
Two and one-half years after initial planning, the HSCT center opened. Twenty transplants were performed during our first year of operation, with an overall survival rate of 100%. This encouraging transplant outcome was attributed to the commitment of a trained multidisciplinary team complemented by partnership with an experienced and dedicated voluntary mentoring team, meticulous planning, adequate equipment and infrastructure, and support from the government and local and expatriate philanthropists.
As of July 16, 2018, an additional 14 patients have received autologous transplants bringing the total number of transplants to 34 within 19 months after opening the HSCT facility at NCI.
We plan to initiate an allogeneic transplantation program at the NCI and to help set up other HSCT centers in Sri Lanka. The SVH team have expressed their commitment to continue mentoring BMT staff at the NCI and NBTS and are prepared to help with the first few allogeneic SCTs as they have with the autologous transplant program.
Acknowledgments
The authors thank the president of Sri Lanka, senior officials of the Health Ministry, and directors of NCI and NBTS; Upulee Madhubhashini, Buddhika Raigamkorale, Dasun Chaminda,Tharaka Elvitigala, Nayana Dadallage, Dammika Priyadarshani, Rathnasiri Bandara, Deepthika Kumari, Sajith Roshan, Ayesha Silva, Sunethra Irangani, Niranjala Ranatunga, and the rest of the staff of the BMT unit at NCI; Helen Tao, Amanda McLaughlin, Annabel Horne, and the staff of the Department of Haematology and Bone Marrow Transplantation at SVH; and Jude Fernando, Chryshanthi Fernando, and the members of the Australian Sri Lanka Society.
This work was supported by The Health Ministry of Sri Lanka, Ahmad Tea (London, United Kingdom), and local and expatriate philanthropic supporters.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: P. Abeysinghe; e-mail: prasadabeysinghe@hotmail.com.