Key Points
Allo-HSCT using reduced intensity conditioning with alemtuzumab appears to be effective and safe for patients with refractory JIA.
Early allo-HSCT may prevent joint damage, reduce toxicity associated with immunosuppression, and reduce transplant-related mortality.
Abstract
Patients with juvenile idiopathic arthritis (JIA) can experience a severe disease course, with progressive destructive polyarthritis refractory to conventional therapy with disease-modifying antirheumatic drugs including biologics, as well as life-threatening complications including macrophage activation syndrome (MAS). Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a potentially curative immunomodulatory strategy for patients with such refractory disease. We treated 16 patients in 5 transplant centers between 2007 and 2016: 11 children with systemic JIA and 5 with rheumatoid factor–negative polyarticular JIA; all were either refractory to standard therapy, had developed secondary hemophagocytic lymphohistiocytosis/MAS poorly responsive to treatment, or had failed autologous HSCT. All children received reduced toxicity fludarabine-based conditioning regimens and serotherapy with alemtuzumab. Fourteen of 16 patients are alive with a median follow-up of 29 months (range, 2.8-96 months). All patients had hematological recovery. Three patients had grade II-IV acute graft-versus-host disease. The incidence of viral infections after HSCT was high, likely due to the use of alemtuzumab in already heavily immunosuppressed patients. All patients had significant improvement of arthritis, resolution of MAS, and improved quality of life early following allo-HSCT; most importantly, 11 children achieved complete drug-free remission at the last follow-up. Allo-HSCT using alemtuzumab and reduced toxicity conditioning is a promising therapeutic option for patients with JIA refractory to conventional therapy and/or complicated by MAS. Long-term follow-up is required to ascertain whether disease control following HSCT continues indefinitely.
Introduction
Systemic juvenile idiopathic arthritis (sJIA) is characterized by arthritis associated with prominent systemic features such as fever, skin rash, serositis, lymphadenopathy, hepatosplenomegaly, and increased inflammatory markers. Clinical outcome is generally poor if symptoms are not well controlled1-2 ; ∼10% of patients with sJIA develop life-threatening complications such as macrophage activation syndrome (MAS), which has a reported mortality of 8% in this context, and/or severe life-threatening infections due to prolonged immunosuppressive treatment with multiple agents.3-6
The main goal of immunosuppressive and anti-inflammatory treatment strategies in sJIA is to achieve disease remission while trying to minimize drug-related toxicity. There are multiple treatment options, including nonsteroidal anti-inflammatory drugs, corticosteroids, disease-modifying antirheumatic drugs (DMARDs) such as methotrexate (MTX), and a variety of biologics targeting key inflammatory cytokines.7 Although most children with sJIA respond to conventional therapy, a small proportion of patients either do not achieve disease remission or experience significant drug-related side effects.8 This can lead to long-term sequelae including permanent joint damage, osteoporosis, growth retardation, and reduced quality of life.7,9 Autologous hematopoietic stem cell transplantation (HSCT) has been assessed as a therapeutic option for children with refractory sJIA since 1997, as an experimental strategy to reset the dysregulated immune system and eliminate autoreactive T cells.9 A number of studies have demonstrated that children undergoing a T-cell–depleted autologous HSCT for refractory JIA, majority with sJIA, can achieve complete drug-free remission.10-14 However, in some cases, disease remission is of transient duration, as a significant proportion of children relapse with inflammatory symptoms after autologous transplant, probably due to reemerging autoreactive T lymphocytes.15 Moreover, T-cell–depleted autologous HSCT is associated with significant mortality, due to prolonged and severe depression of T-cell immunity, leading to viral infections and MAS.11-13
Allogeneic HSCT (allo-HSCT) has potential advantages in this context, with the goal of achieving life-long disease remissions by replacement of the patients’ dysregulated immune system.16-17 Conversely, allo-HSCT introduces the significant risk of graft-versus-host-disease (GVHD). Data on allo-HSCT performed in children with autoimmune diseases and in particular JIA, are extremely scarce.18-20 Here, we report the outcomes of 16 children with severe, refractory JIA who underwent allo-HSCT in 5 different centers between 2007 and 2016.
Methods
Patients and methods
This retrospective observational cohort describes the outcomes of 16 children with refractory JIA: 11 children with sJIA and 5 with rheumatoid factor (RF)-negative polyarticular JIA (poly-JIA) who underwent allo-HSCT in 5 different centers between April 2007 and March 2016. The centers participating in this study were Great Ormond Street Hospital, London, United Kingdom (n = 5); Great North Children’s Hospital, Newcastle upon Tyne, United Kingdom (n = 5); University College Hospital, London, United Kingdom (n = 2); Teaching Hospital Motol, Prague, Czech Republic (n = 1); and Cincinnati Children’s Hospital Medical Center (CCHMC), Cincinnati, OH (n = 3).
Medical charts were reviewed and the following data were extracted from each case using a standardized form: patient demographics, classification of JIA subtype, treatments prior to HSCT, indication for HSCT, donor-recipient matching, conditioning regimen, and graft composition. Posttransplantation data were collected on count recovery, immune reconstitution, lineage-specific chimerism, occurrence of GVHD, infections, and relapse of initial (or occurrence of new, secondary, post-HSCT) autoimmunity. All patients were enrolled in their local institutional review board–approved protocol or treatment plan. Written informed consent was obtained from the parents or legal guardians of all patients prior to the initiation of conditioning therapy for transplantation. Information regarding patient 1 has been previously published.12
Data are presented in terms of frequency and central tendency measures (means and medians). All patients had HLA medium- or high-resolution typing for the following loci: class I (HLA A, B, C) and class II (HLA DQ, DRB1). Patients were considered refractory to treatment if they had active disease that failed to respond to multiple lines of treatment including 2 or more biologics or unacceptable adverse reaction to corticosteroids or biologics.
Complete response (CR) post-HSCT was defined as complete resolution of arthralgia or arthritis and no recurrence of disease symptoms after stopping immunosuppression (IS). Partial response (PR) was defined as: (1) significant clinical improvement and off IS or (2) complete resolution of inflammatory symptoms, but ongoing IS.
Results
Patient characteristics
Patients’ characteristics are summarized in Table 1. The median age at diagnosis was 21.5 months (range, 5 months to 12 years), whereas the median age at transplant was 8.4 years (range, 2.6-16 years). Patients received a median of 5 (range, 2-9) lines of immunomodulatory treatment before HSCT, specifically 9 of them received tocilizumab, and all children were considered refractory to treatment. Five patients had suffered life-threatening complications pre-HSCT. Indication for transplant was: (1) unresponsiveness to standard therapy and biologics (n = 10), (2) failed autologous stem cell transplant (n = 1), or (3) secondary hemophagocytic lymphohistiocytosis (HLH)/MAS, poorly responsive to treatment (n = 5). None of the patients with MAS had a molecular diagnosis of familial HLH.
Transplant procedure
Transplant characteristics are summarized in Table 2. Patients underwent allo-HSCT from a 10 of 10 or 12 of 12 HLA matched unrelated donor (n = 9), 9 of 10 HLA mismatched unrelated donor (n = 3), or 10 of 10 HLA matched sibling donor (n = 4). All patients received a reduced toxicity conditioning regimen with fludarabine 30 mg/m2 per day (from days 7 to 3), melphalan 140 mg/m2 (day 3), alemtuzumab 0.2 mg/kg per day (from days 8 to 4) (n = 10) or fludarabine 30 mg/m2 per day (from days 8 to 4), treosulfan 14 g/m2 per day (from days 6 to 4), alemtuzumab 0.2 mg/kg per day (from days 8 to 4) (n = 6). Of the CCHMC patients, patient 2 received alemtuzumab 0.2 mg/kg per day, days 22 to 18, whereas patients 13 and 14 received it days 14 to 10. All 3 patients received fludarabine 30 mg/m2 per day from days 8 to 4 and melphalan 140 mg/m2 on day 3. Peripheral blood was the stem cell source in the majority of cases (n = 12).
Most patients received cyclosporine (CsA) and mycophenolate mofetil (MMF) as GVHD prophylaxis (n = 10). All patients received antiviral, antifungal, and antibacterial prophylaxis during transplant according to the local policies. The median CD34+ cell dose infused was 9.2 × 106/kg (range, 1.7-32.2 × 106/kg) and the median CD3+ cell dose was 3.2 × 108/kg (range, 0.26-15.5 × 108/kg).
All patients had hematological recovery. The median time to neutrophil recovery >0.5 × 109/L was 13 days (range, 10-65 days), while the median time to platelet recovery >20 × 109/L was 15 days (range, 9-87 days).
With a median follow-up of 29 months (range, 2.8-8 years), 14 of 16 patients were alive: patient 1 died of probable sepsis 20 months posttransplant following an elective procedure to replace the right patella while still on immunosuppressive treatment of chronic GVHD and patient 2 died of invasive fungal infection in the context of grade III aGVHD, 85 days posttransplant, giving a transplant related mortality (TRM) of 12.5%.
TRM
Infections.
The incidence of viral infections/reactivation was high; details are reported in Table 2. Three patients developed shingles, 1 patient experienced Guillain-Barré syndrome associated with varicella zoster virus reactivation and HHV6 enteritis, while 6 patients developed adenoviremia requiring antiviral medications or treatment with adenovirus-specific T cells (patient 4 and patient 11). Two patients developed EBV viremia requiring treatment with rituximab (patients 1 and 9) and 2 patients had CMV reactivation leading to pneumonitis in 1 case (patient 3). One patient developed BKV encephalitis (patient 1). There was 1 case each of Serratia fasciitis (patient 11) and of infection with atypical mycobacteria (patient 3). Two patients experienced severe bacterial infections and 1 child presented disseminated life-threatening fungal disease (patient 2).
GVHD.
Three patients experienced grade II-IV acute GVHD (aGVHD). There was 1 patient with extensive chronic GVHD (patient 1) at the last follow-up. On balance, patient 12 and patient 16 were considered to have recurrent disease post-HSCT as both presented with active polyarthritis, but distinguishing recurrent disease from chronic GVHD in this setting can be very difficult.
JIA outcome and donor engraftment.
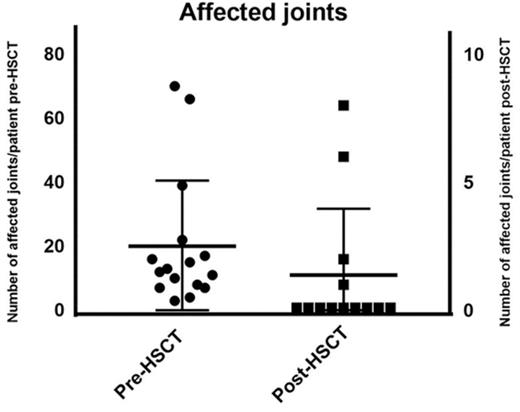
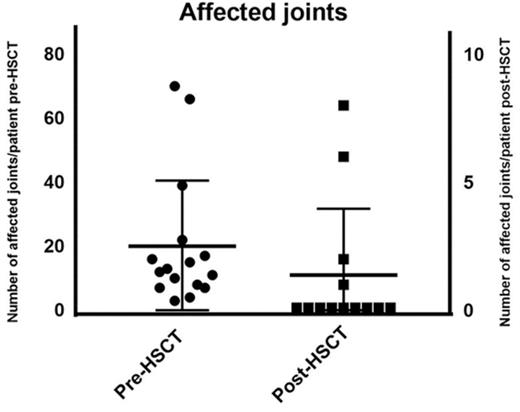
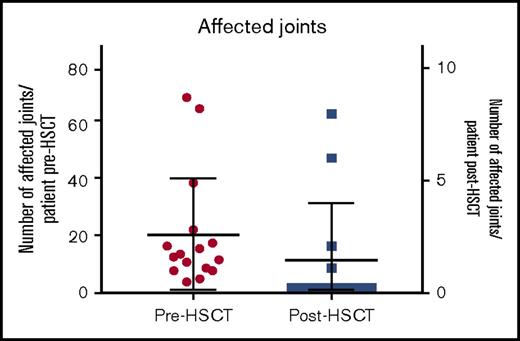
The median number of affected joints pretransplant was 12 (range, 3-70 joints) whereas posttransplant 4 patients had active joint inflammation: patient 5 had a reduced number of joints affected (feet, knees, hands, and shoulders) compared with 39 joints affected pretransplant; patient 9 had 1 active joint transiently; patient 12 had a reduced number of joints from almost all joints affected to 8 joints and patient 16 had polyarthritis following cessation of IS as shown in Figure 1. At last follow-up, 11 of 14 surviving patients were in complete remission (symptom-free and off immunosuppressants), 2 of 4 surviving patients with poly-JIA and 9 of 10 with sJIA. Patient 5 achieved complete remission posttransplant sustained after cessation of IS but had relapse of arthritis and uveitis 5 years post-HSCT requiring intra-articular corticosteroids and tocilizumab. Patient 12 had chronic severe active disease prior to HSCT, which was refractory to immunosuppressive treatment: she was immobile, fully dependent on her mother, and unable to take care of herself. After transplant, she experienced a significant clinical improvement: at last follow-up, she was physically active, walking and with no pain, although she was still dependent on corticosteroids and MMF. Patient 9, who had sJIA and MAS prior to his HSCT, presented 22 months following the allo-HSCT with fevers, skin rash, and arthralgia in 1 joint. This was treated with a brief course of corticosteroids, with complete resolution of the symptoms. He was in CR and off immunosuppressant at last follow-up. Patient 16 developed synovitis as corticosteroids were tapered 2 months post-HSCT but responded to an increased dose of corticosteroids; at 6 months post-HSCT, as CsA was stopped, she presented with fevers, polyarthritis, cytopenias (all 3 lineages), raised ferritin, and inflammatory markers but did not 100% fulfill the MAS criteria. At the same time, she dropped the percentage of naive T cells with significant increase in activated DR+ T cells, although with high levels of donor chimerism in CD15 and CD3 lineages. She was treated with pulsed methylprednisolone, high-dose immunoglobulin, rituximab, and anakinra with relatively good response but ongoing joint inflammation.
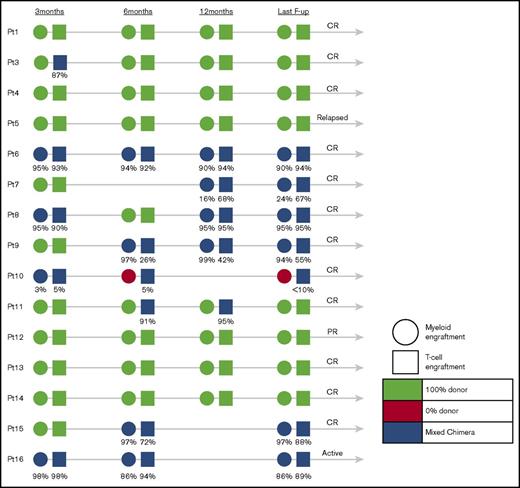
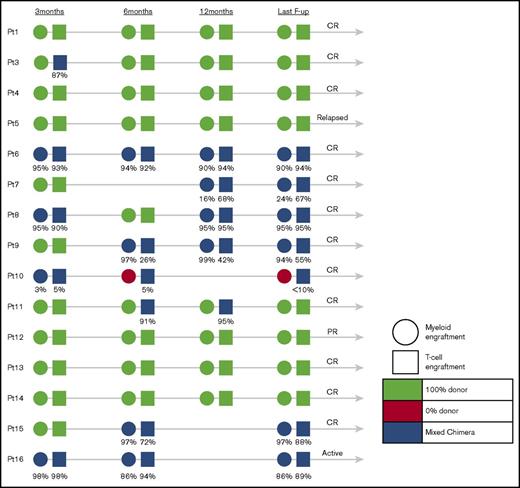
Results of donor engraftment were available in 15 of 16 children (Figure 2). Eight children achieved 100% donor engraftment in peripheral blood, whereas 6 patients who had chimerism monitored in different cell lineages (T cells, B cells, myeloid cells) expressed very high levels of donor engraftment in their T-cell compartment (median, 86%; range, <10%-95%) and myeloid compartment (median, 90%; range, 0%-97%). There was no obvious correlation between the level of myeloid or T-cell donor engraftment and disease outcome, exemplified by patient 12, who in spite of 100% donor engraftment achieved only PR, patient 5 who despite of 100% donor engraftment relapsed late post-HSCT, patient 16 who relapsed after stopping IS despite high levels of donor engraftment in both lymphoid and myeloid lineages, and patient 10 who remains in CR in spite of 0% myeloid and only low-level T-cell donor engraftment.
Engraftment of patients who underwent allogeneic stem cell transplantation at 3, 6, and 12 months and at the last follow-up.
Engraftment of patients who underwent allogeneic stem cell transplantation at 3, 6, and 12 months and at the last follow-up.
The median time to the withdrawal of IS was 7 months (range, 4-24 months).
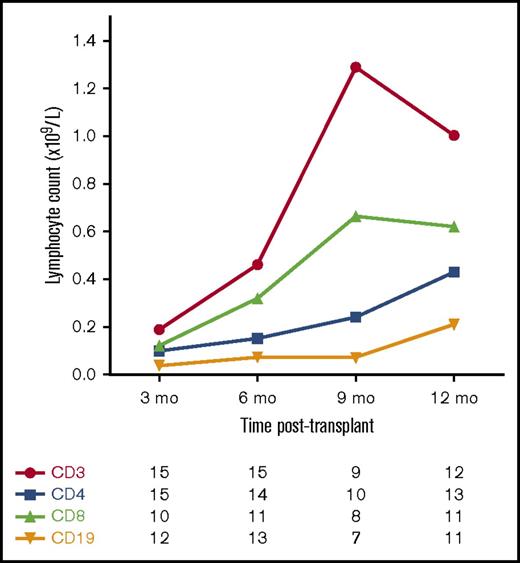
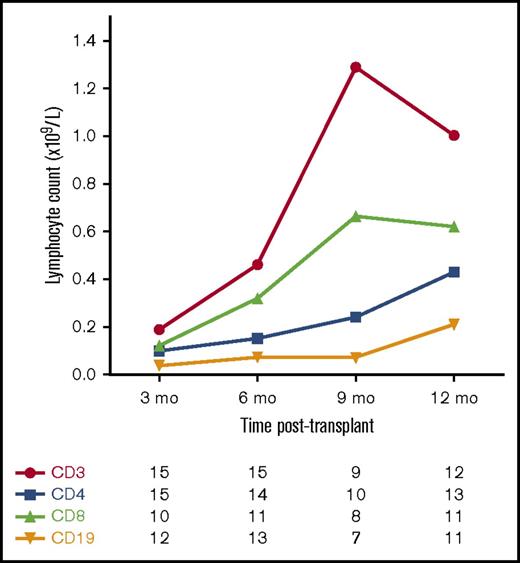
Figure 3 shows the pattern of T-cell and B-cell reconstitution after transplant. T-cell reconstitution was generally slow, with a median time to achieve a CD4+ T-cell count ≥0.3 × 109/L of 12 months (range, 6-15 months).
Median T-cell and B-cell counts following allo-HSCT for JIA. Number of patients with available data at each time point.
Median T-cell and B-cell counts following allo-HSCT for JIA. Number of patients with available data at each time point.
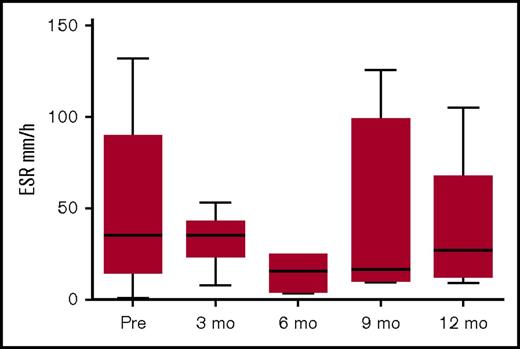
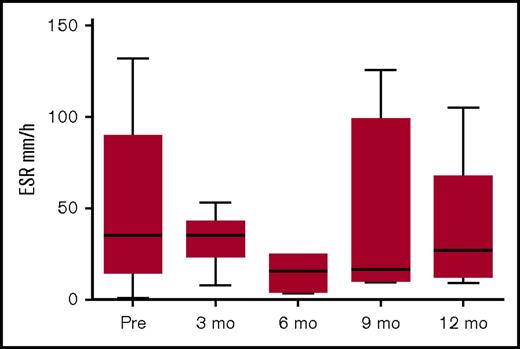
Figure 4 shows the pattern of erythrocyte sedimentation rate (ESR) pre- and post-allo-HSCT. The levels pre-HSCT were not very high, probably due to immunomodulation pretransplant. Although not all centers assessed ESR following HSCT, there was a trend to reduced ESR post-HSCT.
ESR pre- and post-HSCT. Data available for 15 patients pre-HSCT; for patients 1, 2, 3, 9, 11, and 16 at 3 months post-HSCT; for patients 1, 6, 9, and 11 at 6 months post-HSCT; for patients 1, 2, 8, 9, and 11 at 9 months post-HSCT, and for patients 1, 2, 6, 9, and 11 at 12 months post-HSCT.
ESR pre- and post-HSCT. Data available for 15 patients pre-HSCT; for patients 1, 2, 3, 9, 11, and 16 at 3 months post-HSCT; for patients 1, 6, 9, and 11 at 6 months post-HSCT; for patients 1, 2, 8, 9, and 11 at 9 months post-HSCT, and for patients 1, 2, 6, 9, and 11 at 12 months post-HSCT.
Autoimmunity.
Five patients developed secondary autoimmunity posttransplant, mainly cytopenias, and were responsive to short courses of IS. Patient 3 developed severe pancytopenia requiring 2 CD34+ cell infusions, as well as IVIG, rituximab, eltrombopag and eventually splenectomy; patient 13 developed immune thrombocytopenia requiring rituximab, whereas patient 8 experienced immune-mediated neutropenia and thrombocytopenia treated with IVIG, corticosteroids, rituximab, and MMF.21 Patient 5 developed severe but transient Guillain-Barré syndrome. Patient 15 developed transient thyrotoxicosis evolving to hypothyroidism.
Discussion
Children affected by JIA can experience significant morbidity associated with poorly controlled disease and long-term immunosuppressive treatment, in particular serious infections, neuropsychiatric adverse events, autoimmunity, and an increased risk of malignancy.3,5,22-25
Over the past decade, the experience with autologous stem cell transplantation (ASCT) for patients with refractory JIA has produced valuable data. The biological mechanisms by which ASCT works were shown to be not only by the eradication of autoreactive lymphocytes with immunosuppressive conditioning and T-cell depletion, but also importantly by restoration of the chimeric T-cell receptor (TCR) repertoire and CD4+CD25+ T regulatory cells.26-29 Rates of drug-free remission were between 50% and 57%.13,30 However, in a prospective follow-up of the initial multicenter study,11 12 of 20 patients relapsed between 2 and 16 months posttransplant and 1 patient relapsed 7 years following ASCT.12 The mechanism of relapse was hypothesized to have occurred due to insufficient lymphoablation and clonally skewed TCR repertoire26 or due to recurrence of activated effector CD4+ T lymphocytes (autoreactive lymphocytes).15 Moreover, the initial mortality associated with the procedure was high at 9% as 2 patients died of MAS early post-ASCT11 and a similar high mortality rate was reported at a later stage from the United Kingdom experience.13 This high TRM was attributed to the combination of significant T-cell depletion and infection.11-13,18
Considering that the pathogenesis of sJIA is very similar to known genetic autoinflammatory diseases affecting mostly the innate immune system,31-32 there might be a theoretical advantage of allogeneic stem cell transplant compared with ASCT. Allogeneic stem cell transplant in animal models has been shown to generate intrathymic tolerance more effectively when compared with autologous transplantation.33 Moreover, allogeneic stem cell transplantation has the potential for a graft-versus-autoimmune effect,16,33 documented in a series of patients who underwent HSCT for hematological conditions and concomitant autoimmune diseases.34-39 Somewhat unexpectedly, the results of both the European experience (1984-2007)40 and a United Kingdom national analysis (1997-2009) of allo-HSCT for severe autoimmune diseases showed 55% CR and 23% PR in the European database, and sustained responses in 60% to 70% of patients undergoing allo-HSCT compared with 30% to 40% of patients undergoing autologous HSCT in the United Kingdom database.18
Following our initial report,19 we describe the outcomes of reduced intensity allogeneic transplantation for JIA in a multicenter cohort of 16 pediatric patients in a 9-year period. With the follow-up duration ranging from 85 days to 8 years, we observed an overall survival of 87.5%. At last follow-up, 11 of 14 surviving patients were in CR (symptom free and off immunosuppressants), 1 was in PR, and 2 had active disease (1 late relapse and 1 relapse after cessation of immunosuppressants).
In this complex cohort of patients where allo-HSCT was performed relatively late in the course of the disease, TRM was expectedly high at 12.5%, but not dissimilar to the TRM reported for autologous T-cell–depleted HSCT.11,13 There was a high incidence of viral infections and/or viral reactivation, not unexpected in the context of in vivo T-cell–depleted transplantation. We did observe the occurrence of secondary autoimmunity post-HSCT, mainly cytopenias, which did not require prolonged treatment as has been previously reported.41
Selecting eligible patients for allo-HSCT earlier in the course of their progressive disease rather than leaving it as the “last resort”42-43 could significantly improve the outcome, in particular by reducing morbidity from potentially life-threatening infections and preventing the development of severe joint damage and consequent complications.
For those rare patients presenting with potential life-threatening complications such as pulmonary hypertension and/or MAS44 the indication may be more straightforward. However, for patients with otherwise uncomplicated but refractory disease, that is, those not responding to multiple conventional or biologic DMARDs, defining the correct indications for allogeneic transplantation remains a challenge.32 At the present time there are several studies assessing potential biomarkers and specific molecular defects associated with a severe disease phenotype and response to therapy.45-46 While those are validated, indication for HSCT is defined individually and according to the experience of the center, as this approach is still an experimental therapy.42,47 Normalization of raised serum interleukin-18 (IL-18) levels has recently been demonstrated posttransplant, hence serum IL-18 may potentially be a useful biomarker to assess disease activity of sJIA in this context.48-49
Of note, a potential disadvantage of allo-HSCT following a failed autologous procedure is the possibility of adding comorbidities, especially from severe infections (eg, patient 1). The indications for and the role of allo-HSCT for treating children with JIA may be better defined once genetic defects or biomarkers associated with severe phenotypes of the disease are revealed.
Despite reduced intensity conditioning, all patients engrafted donor cells in all cell lineages except 1 patient who underwent a mismatched unrelated donor transplant and only engrafted donor T cells. It is not known at this stage whether there will be an impact of mixed chimerism on the ongoing remission status of the disease posttransplantation. In this cohort of patients, full donor chimerism was not always associated with remission: 1 patient with 100% donor cell engraftment had only partial response with ongoing inflammation of big joints requiring low-dose corticosteroids and MMF 12 months posttransplant, 2 patients with high-level donor cell engraftment have active disease at last follow-up (1 relapsed late posttransplant [patient 5] and 1 patient relapsed following cessation of IS 6 months posttransplant), whereas another patient with only partial T-cell engraftment remains in CR 17 months posttransplant. The mechanism of remission in the latter patient is possibly due to self-tolerance of autoreactive T cells, mirroring that in the autologous HSCT setting.27 Similar to the results from the ASCT experience, we presume that significant joint damage prior to transplant is not reversible with the procedure even despite full donor chimerism.15
As this was an observational retrospective study, there were limitations relating to the availability of data to fully assess JIA disease activity pre- and post-HSCT using the core set criteria.50 For this study, we were only able to collect data on joint counts and ESR (Figures 1 and 4, respectively).
In conclusion, we show that reduced intensity HSCT for severe, refractory JIA is a safe and effective procedure to rescue patients that did not respond despite being heavily treated, or those who developed complications associated with MAS. However, to reduce TRM and morbidities, mainly infections, we favor considering early HSCT for those eligible patients with a suitable available donor avoiding prolonged use of immunosuppressive medications and preventing severe and potentially irreversible joint damage, and/or treatment-associated burden of disease mainly growth retardation, hypertension, and osteoporosis due to prolonged corticosteroid usage. Prospective studies are warranted to harmonize selection of patients and indications for transplant, to evaluate conditioning regimens that could promote robust engraftment with even lower toxicity, to assess potential biomarkers, and to investigate immunological mechanisms of remission following allo-HSCT.
Clearly, longer follow-up is required to confirm the effectiveness of allo-HSCT for severe, refractory JIA as late relapse has been observed in this setting. As JIA is a complex group of disorders, it is of paramount importance that the follow-up post-HSCT includes a multidisciplinary team (transplant, rheumatology, immunology, and physiotherapy specialties) following standardized methods. This would facilitate better assessment of disease response, better evaluation of the role of HSCT in the therapeutic algorithm, and indicate whether there is any difference in the outcomes between the subtypes of the disease.
Acknowledgments
The authors thank Yasmine Koodun for assistance providing patient data.
This work was supported by the National Institute for Health Research (NIHR) Great Ormond Street Hospital Biomedical Research Centre.
The views expressed are those of the author(s) and not necessarily those of the National Health Service (NHS), the NIHR, or the Department of Health.
Authorship
Contribution: J.M.F.S., M.A. and P.V. designed the study, analyzed data, and wrote the paper; and F.L., B.C., S.C., P.S., R.F., V.G., M.F., A.J.C., Z.N., M.A.S., A.R.G., S.H., T.J.F., G.L., R.C., K.R., P.J.A., P.B., L.R.W., J.M.G., R.H., and R.M. provided clinical care, collected and submitted data, and contributed to the final draft of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Juliana M. F. Silva, Department of Bone Marrow Transplantation, Great Ormond Street Hospital for Children NHS Trust, Great Ormond St, London WC1N 3JH, United Kingdom; e-mail: juliana.silva@gosh.nhs.uk.