Key Points
Obinutuzumab preinduction results in abrogating high TLS risk in patients with CLL.
Venetoclax and obinutuzumab therapy results in high rates of undetectable MRD.
Abstract
Early data on venetoclax-containing regimens for the treatment of chronic lymphocytic leukemia (CLL) show promising results with deep remissions, but are hampered by potential risk for tumor lysis syndrome (TLS). Whether optimal duration of venetoclax treatment can be guided by minimal residual disease (MRD) is currently unknown. To study whether TLS risk can be mitigated in an unfit population by introducing preinduction, and whether MRD-guided duration of venetoclax treatment is a feasible and efficacious approach, we performed the Dutch-Belgian Cooperative Trial Group for Hemato-oncology (HOVON) 139/GIVE trial. The study treatment consists of 4 treatment phases: preinduction (2 cycles obinutuzumab), induction I (6 cycles obinutuzumab and venetoclax), induction II (6 cycles venetoclax), and a randomization phase (group A: maintenance with 12 additional cycles of venetoclax irrespective of MRD; group B: MRD guided venetoclax maintenance with a maximum of 12 cycles). Here we report on a planned interim safety analysis as well as preliminary efficacy and MRD data of the first 30 patients enrolled. Downgrading of TLS risk after preinduction occurred in 25 patients: 3 from high to medium, 3 from high to low, and 19 from medium to low risk. No patient remained high risk. From these 30 patients, peripheral blood MRD data were obtained for 28 patients at the end of induction II (6 months after the last obinutuzumab dose), of whom 26 had undetectable MRD levels, and for 18 patients who reached the 3-month after-randomization point, of whom 16 had undetectable MRD levels. Obinutuzumab preinduction is tolerated well in these unfit patients and results in abrogating high TLS risk in all patients. Preliminary data indicate that efficacy is maintained with a high proportion of patients with undetectable MRD levels after combination treatment.
Introduction
First-line treatment of patients with chronic lymphocytic leukemia (CLL) unfit for fludarabine-containing chemoimmunotherapy with the Bruton tyrosine kinase–inhibitor ibrutinib proved superior to chlorambucil monotherapy in terms of both progression-free survival and overall survival. Yet the frequency of complete remissions remained low, eradication of minimal residual disease (MRD) was not seen, and ibrutinib needs to be continued until disease relapse or unacceptable toxicity.1 Obvious disadvantages of continuous treatment are long-term exposure to at least partly unexplored adverse effects, high costs, and the inevitable selection of resistant clones. Therefore, novel safe regimens are needed that result in deep and durable responses allowing for early treatment cessation.
Venetoclax is a highly selective inhibitor of the anti-apoptotic protein B-cell lymphoma 2, which is constitutively overexpressed in CLL.2 Venetoclax monotherapy has shown high efficacy in patients with heavily pretreated CLL.3 A recent study showed that a fixed duration of venetoclax treatment combined with the anti-CD20 monoclonal antibody rituximab results in undetectable levels of MRD in the majority of relapsed/refractory patients.4 Although yet to be proven for drugs such as venetoclax, MRD levels posttreatment proved to be a robust surrogate endpoint for long-term outcome after chemoimmunotherapy.5,6 It is anticipated that the combination of venetoclax with the more potent anti-CD20 antibody obinutuzumab may allow for MRD-guided instead of fixed-duration treatment, allowing a lower incidence of toxicity, as well as lower costs.
Nonetheless, specific toxicities of these 2 drugs might limit their applicability in a fludarabine-unfit population. First, venetoclax might result in potentially life-threatening tumor lysis syndrome (TLS), specifically in patients with high tumor burden and impaired kidney function,7 and necessitates frequent admissions in patients at high risk.3 Second, occurrence of severe infusion-related reactions (IRRs) has been reported during obinutuzumab infusion, especially at the first dose.8 The current phase 3 trial of the German CLL study group (CLL14) compares chlorambucil combined with obinutuzumab with venetoclax and obinutuzumab as first-line treatment in patients with CLL with significant comorbidities. In this protocol, venetoclax is added to obinutuzumab on the 22nd day of the first treatment cycle. A recently reported safety and efficacy analysis of the first 12 patients included in the run-in phase of the CLL14 trial showed grade 3 laboratory TLS in 2/12 patients (16.7%), 1 during venetoclax ramp-up, and occurrence of obinutuzumab-related IRRs in 9/12 patients (75.0%) during the first obinutuzumab administration.9
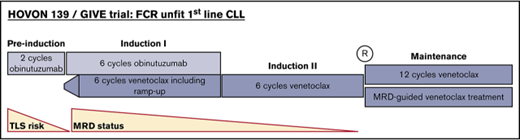
Within the Dutch-Belgian Cooperative Trial Group for Hemato-oncology (HOVON) study group, we currently perform the randomized phase 2 HOVON 139/GIVE trial (Netherlands Trial Registry ID number #NTR604) to address whether venetoclax-mediated TLS risk can be mitigated in an fludarabine-unfit population by introducing 2 preinduction cycles of obinutuzumab, and whether MRD-guided duration of venetoclax treatment instead of a fixed-duration regimen is a feasible and effective approach.
With permission of the independent data safety monitoring board, we here report on a planned interim safety analysis that is focusing on the safety of the debulking strategy with preinduction. Results of the first cohort of 30 (eligible) patients who received the first 3 cycles (2 preinduction cycles, 1 cycle of induction I; see “Methods”) were presented to the data safety monitoring board. We also report on preliminary efficacy with blood and bone marrow MRD analyses of these patients.
Methods
Patient selection
For this phase 2 study, previously untreated patients with CLL with indication for treatment according to International Working Group on CLL 2008 criteria10 were enrolled if they were considered unfit for treatment with the combination of rituximab, fludarabine, and cyclophosphamide by their treating physician. A complete list of inclusion and exclusion criteria is presented in supplemental Data 1. The study was approved by the Medical Ethical Committee of the Academic Medical Center in Amsterdam. Written informed consent was given by all patients. Cumulative illness rating scale (CIRS) scores were obtained locally, and immunoglobulin heavy chain rearrangement and cytogenetics, TP53 mutation status, and complex karyotype (defined as ≥3 aberrations, obtained by comparative genomic hybridization array) were obtained centrally at the Academic Medical Center in Amsterdam. The number of patients scheduled to be included is 70, and this manuscript is based on a preplanned safety interim analysis on the first 30 (eligible) patients.
Treatment
The study treatment consists of 4 treatment phases. First, patients receive preinduction with 2 cycles of obinutuzumab monotherapy (C1 d1: 100 mg; d2: 900 mg; d8: 1000 mg; d15: 1000 mg; C2 d1: 1000 mg). Details of premedication (acetaminophen, an antihistamine, and steroids) given before obinutuzumab treatment are described in supplemental Data 2. The next phase, induction I, consists of combination therapy with 6 cycles obinutuzumab (C3-8 d1: 1000 mg) and venetoclax (C3: ramp-up weekly 20, 50, 100, and 200 mg, and 400 mg daily thereafter), which is followed by single-agent treatment with 6 cycles venetoclax monotherapy (C9-14: 400 mg daily), induction II. After that, patients who are at least in partial remission are randomly assigned between group A: maintenance treatment with 12 additional cycles of venetoclax irrespective of MRD, and group B: MRD-guided venetoclax maintenance. All cycles have a duration of 28 days. Primary endpoint of the study is MRD-negative bone marrow after maximum 24 cycles of (planned) venetoclax and no progression according to International Working Group on CLL criteria at any earlier point.
TLS risk
TLS risk is scored using the 3 categories (high: either absolute lymphocyte count [ALC] ≥25 × 109/L and largest diameter of all measurable lymph nodes ≥5 cm and <10 cm or largest diameter of all measurable lymph nodes ≥10 cm, regardless of the ALC; intermediate: either ALC ≥25 × 109/L or largest diameter of all measurable lymph nodes ≥5 cm and <10 cm; low: ALC <25 × 109/L and largest diameter of all measurable lymph nodes < 5 cm), as earlier described3 and shown in supplemental Table 1. TLS risk was assessed by both ALC and CT scan before and after preinduction treatment with 2 courses of single-agent obinutuzumab. TLS risk mitigation steps were performed as described in supplemental Data 3, similar to venetoclax development studies4,11 and according to European summary of product characteristic/US prescribing information.
MRD
MRD on blood is centrally analyzed after induction I (C8, after combination treatment, 4 weeks after last obinutuzumab infusion), after induction II (C14, after 6 additional cycles of venetoclax monotherapy and 6 months after the last obinutuzumab infusion), and at the 3-month after-randomization point (9 months after the last obinutuzumab infusion). MRD is quantified by 6-color flow cytometry with a sensitivity of at least 10−4, as previously validated against allele-specific oligonucleotide–primer real-time quantitative immunoglobulin heavy chain polymerase chain reaction,12 and uses an international standardized approach.13 MRD values are categorized into 3 different MRD levels: undetectable (<10−4; ie, less than 1 CLL cell per 10 000 leukocytes), intermediate-positive (≥10−4 and <10−2), and high-positive (≥10−2). In addition, MRD on bone marrow is centrally analyzed after induction II (C14).
Statistical considerations
Sample size and power calculation.
In this phase 2 study, each (maintenance) treatment group will be analyzed separately. As such, the study can be seen as 2 parallel studies, and no formal comparison between the treatment groups will be made. Sample size was calculated using an A’hern design, with P0 being the largest success rate, which, if true, implies that the therapeutic activity is too low, and therefore does not warrant further investigation, taken as 25%; and P1 being the smallest success rate, which, if true, implies that the therapeutic activity is sufficiently high and therefore warrants further investigation in clinical trials, taken as 50%. To reject the null hypothesis, 26 eligible patients are required in each maintenance treatment group. In the case at least 11 successes are observed out of these 26 patients, further investigation is warranted. Taking into account that 2 parallel maintenance treatment groups will be analyzed, as well as the expected number of patients not reaching at least partial remission, and therefore not receiving maintenance (about 20%), 65 patients have to be registered in the study. However, to overcome dropout resulting from ineligibility, 70 patients will be included in the trial.
We now report on the preplanned safety interim analysis; no results on the primary endpoint are presented here. In addition, preliminary data on MRD development are presented.
Results
Clinical characteristics of patients
Thirty patients who were followed for at least 3 cycles are included in this analysis. The median age was 70 years (range, 57-79 years), the majority of patients were male (73%), 47% had Rai stage ≥3, 50% had an unmutated immunoglobulin heavy chain rearrangement, and median CIRS score was 3 (range, 0-16) and mean creatinine clearance was 77 mL/min (range, 45.0-116 mL/min). Deletion 17p was found in 3 (10%) patients; in 7 (24%) patients, a TP53-mutation (≥10%) was found, and 9 (30%) had complex karyotype (≥3 cytogenetic abnormalities), as depicted in Table 1.
Impact of obinutuzumab preinduction on TLS risk score
Baseline as well as TLS risk scores after 2 cycles of obinutuzumab are summarized in Table 2. Downgrading of TLS risk after 2 cycles preinduction with obinutuzumab occurred in 25 patients (83%): 3 from high to medium risk, 3 from high to low risk and 19 from medium to low risk. No patients remained high risk.
This TLS downgrading was mainly a factor of absolute lymphocyte count (ALC) decrease and, to a lesser extent, of reduction of largest diameter of all measurable lymph nodes. Figure 1A shows that in all patients with an ALC above the TLS risk threshold of 25 × 109/L at baseline (n = 27), their ALC decreased below this threshold after 2 cycles of preinduction with obinutuzumab with a median of ALC of 1.20 (range, 0.30-2.60) after 1 cycle of preinduction and 1.40 (range, 0.50-3.90) after 2 cycles of preinduction. In the majority of patients, preinduction treatment with obinutuzumab resulted in reduction of the largest diameter of lymph nodes and 4 changed in lymph node risk threshold (1 patients from ≥10 cm to nodes ≥5 cm and <10 cm, and 3 patients from nodes ≥5 cm and <10 cm to <5 cm), as depicted in Figure 1B. Combining these data, a per patient change in TLS risk category is shown in Figure 1C.
TLS risk mitigation after 2 preinduction cycles with obinutuzumab. (A) ALC at baseline and at end of preinduction per patient. (B) Largest diameter of lymph node in millimeters, as assessed by CT scan at baseline and at end of preinduction per patient. (C) Per patient change in TLS risk category; 3 = high (ALC ≥25 × 109/L and largest diameter of all measurable lymph nodes ≥5 cm and <10 cm or largest diameter of all measurable lymph nodes ≥10 cm, regardless of the ALC), 2 = intermediate (ALC ≥25 × 109/L, or largest diameter of all measurable lymph nodes ≥ 5 cm and <10 cm), 1 = low (low: absolute lymphocyte count (ALC <25 × 109/L and largest diameter of all measurable lymph nodes <5 cm).3
TLS risk mitigation after 2 preinduction cycles with obinutuzumab. (A) ALC at baseline and at end of preinduction per patient. (B) Largest diameter of lymph node in millimeters, as assessed by CT scan at baseline and at end of preinduction per patient. (C) Per patient change in TLS risk category; 3 = high (ALC ≥25 × 109/L and largest diameter of all measurable lymph nodes ≥5 cm and <10 cm or largest diameter of all measurable lymph nodes ≥10 cm, regardless of the ALC), 2 = intermediate (ALC ≥25 × 109/L, or largest diameter of all measurable lymph nodes ≥ 5 cm and <10 cm), 1 = low (low: absolute lymphocyte count (ALC <25 × 109/L and largest diameter of all measurable lymph nodes <5 cm).3
Toxicities
No clinical TLS occurred. Laboratory TLS grade 1 according to Cairo Bishop criteria14 occurred during preinduction with obinutuzumab in 2 patients with medium TLS risk (twice in 1 patient). Laboratory TLS grade 1 also occurred in 2 patients during venetoclax ramp-up, of whom 1 had high and 1 had medium TLS risk at baseline, but both had low TLS risk after preinduction. All cases of TLS resolved with protocolled intravenous hydration and allopurinol ± rasburicase (supplemental Data 3).
During the first preinduction cycle, 9 (30%) patients experienced grade 3 to 4 toxicities (all IRR), but none in the second preinduction cycle, and all these patients were able to continue further obinutuzumab treatment. The intended dose of 3000 mg obinutuzumab in the first preinduction cycle was administered in 28 patients (93%). During first induction cycle (combination therapy of obinutuzumab and venetoclax), 9 (30%) patients developed a grade 2 toxicity and 3 (10%) developed a grade 3 toxicity (all neutropenia). All patients received total intended dose of venetoclax ramp-up.
Preliminary MRD analyses
For the 30 patients included in the safety interim analysis, we performed serial MRD testing on available samples (Figure 2A). Peripheral blood MRD data were obtained for 29 patients who had reached the end of induction I (end of combination treatment, so only 4 weeks after last obinutuzumab infusion, with potential risk for false-negative MRD results5 ), for 28 patients who had reached the end of induction II (venetoclax monotherapy, 6 months after end of the immunotherapy anti-CD20 by obinutuzumab), and for 18 patients who reached the point of 3 months after randomization. At end of induction I, 24 patients (80%) became MRD-undetectable, of whom 6 with high-risk genetics (del[17p] and/or TP53 mutation). One patient remained MRD high-positive; 4 became MRD intermediate-positive. MRD measurement at end of induction II (6 months after last obinutuzumab infusion) showed that all patients with MRD-undetectable disease remained MRD-undetectable. MRD results of those 5 patients with detectable MRD after induction I were as follows after completion of induction II: the patient with MRD high-positive disease became MRD intermediate-positive, and of the 4 patients with MRD intermediate-positive disease, 1 remained intermediate-positive and 3 became MRD-undetectable. Of the first 18 patients who reached the point of 3 months after randomization, 16 were MRD-undetectable and 2 had MRD intermediate-positive disease. One of these, 2 MRD intermediate-positive patients became MRD positive after being MRD-undetectable at the end of induction II, and 1 was also MRD intermediate-positive at end of induction II. Of the 16 patients with MRD-undetectable disease at 3 months after randomization, 1 had still MRD (intermediate-positive level) at the end of induction II and per protocol continued venetoclax, and 15 had undetectable MRD levels at end of induction II and were randomly assigned per protocol to continue/stop venetoclax. The kinetics of MRD per patient are shown in Figure 2B.
Serial MRD results. MRD data were obtained for 29 of 30 patients at the end of induction I (combination treatment) and 28 of 30 patients at the end induction II (venetoclax monotherapy), cycle 8 and cycle 14, respectively. Additional MRD data were obtained for 18 of 30 patients at 3 months after randomization. (A) Absolute numbers of measured MRD on peripheral blood. (B) Kinetics of MRD per patient.
Serial MRD results. MRD data were obtained for 29 of 30 patients at the end of induction I (combination treatment) and 28 of 30 patients at the end induction II (venetoclax monotherapy), cycle 8 and cycle 14, respectively. Additional MRD data were obtained for 18 of 30 patients at 3 months after randomization. (A) Absolute numbers of measured MRD on peripheral blood. (B) Kinetics of MRD per patient.
Bone marrow MRD results were obtained in 26 patients at the end of induction II. Of the 26 patients, 24 were concordant with the peripheral blood MRD. Total MRD results per point are shown in Figure 2A.
Discussion
Our preliminary results demonstrate that in fludarabine-unfit patients, preinduction with obinutuzumab monotherapy is well tolerated and results with significant downgrading of TLS risk, whereas efficacy of venetoclax plus obinutuzumab regimen is maintained, as seen with a high rate of MRD-undetectable disease. In preinduction, grade 3 to 4 IRRs occurred in 30% of cases despite premedication, but the large majority of these patients were able to receive the full dose of obinutuzumab; no fatal IRRs were seen.
Preinduction cycles abrogated high TLS risk in all patients, which could potentially affect the way patients are treated in outpatient setting, as opposed to inpatient. Yet, laboratory TLS grade 1 did occur during venetoclax ramp-up in 2 patients, of whom 1 with high and 1 with medium TLS risk at baseline, but both with low TLS risk after preinduction. This observation indicates that despite downgrading in TLS risk, prophylaxis and mitigation for TLS should be continued. As matter of fact, it would be advisable to collect data on TLS obtained during trials (and ideally also real-life observations) into a database that should also include patient characteristics to determine an improved TLS scoring algorithm.
Also, this study shows that with the regimen used, efficacy was maintained and remained unchanged compared with previous reports. Undetectable MRD occurs in the vast majority of patients after 6 months of combination treatment and is sustained 6 months after last obinutuzumab administration and 9 months after end of obinutuzumab (3 months after randomization). This is remarkable, as the flow-based MRD assay during or shortly after anti-CD20 therapy, specifically when performed on peripheral blood, can have false-negative results. The fact that our preliminary data indicate a high concordance between blood and bone marrow MRD levels at end of induction II might indeed suggest that this regimen, when given as first-line treatment, results in deep remissions in both compartments, even in patients with high-risk genetics and complex karyotype. Further treatment and observation are warranted to assess both full TLS frequency and efficacy of MRD-guided venetoclax treatment duration objectives of this patient population. At this interim follow-up, we saw that despite being deemed unfit for fludarabine by their treating physician, patients had an overall CIRS score of 3 and had an overall normal creatinine clearance. Therefore, these safety data should be used with caution for true frail patients until full data from the study are available.
In conclusion, obinutuzumab preinduction followed by 6 cycles of obinutuzumab and venetoclax and 6 cycles of venetoclax monotherapy is well tolerated, results in mitigation of TLS risk, and might result in a very high percentage of undetectable MRD. The data safety monitoring board advised to continue the study as planned.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors gratefully thank the patients who participated and their families.
This study is sponsored the by HOVON study group on behalf of the HOVON CLL working group. Data were analyzed by the HOVON data center at the Erasmus University Medical Center in Rotterdam, and the authors thank all team members involved. This study was supported by F. Hoffmann-La Roche Ltd.
Authorship
Contribution: A.P.K., S.K., and M.-D.L. designed the research; collected, analyzed, and interpreted the data; and wrote the paper; J.D. analyzed and interpreted the data and wrote the paper; Y.v.N. designed the research and analyzed and interpreted the data; J.A.D., C.H.M., and L.M.E. analyzed data; F.C.-d.B., J.S., E.v.d.S., H.V., C.I., S.W., M.H., and S.H.T. provided patients and collected data; M.M. designed the research; and all authors critically reviewed the paper and approved the final version.
Conflict-of-interest disclosure: A.P.K. received research funding from and participated in ad boards from Roche/Genentech and AbbVie. M.M. is a Genentech employee. The remaining authors declare no competing financial interests.
A complete list of the members of the HOVON CLL study group appears in the supplemental appendix.
Correspondence: Arnon P. Kater, Department of Hematology, Amsterdam University Medical Centers, University of Amsterdam, 1105 AZ Amsterdam, The Netherlands; e-mail: a.p.kater@amc.uva.nl.