Key Points
Patients who received R-chemo followed by MR had an improved 5-year PFS and OS independent of prognostic factors.
A reduction of the risk of HT was observed among the MR patients of the training, but not of the validation, cohort.
Abstract
The introduction of the anti-CD20 antibody rituximab in combination with chemotherapy (R-chemo) has improved the prognosis of patients with follicular lymphoma (FL). During the last decade, the addition of a maintenance treatment with rituximab (MR) after R-chemo has been tested with the hope of further improving the outcome of these patients. Using 2 independent population-based cohorts, we investigated the effect of up-front MR on time related end points as well as the risk of histological transformation (HT). FL patients were included if they: (1) completed first-line induction treatment with R-chemo, (2) were alive after induction treatment and eligible for MR, and (3) had no evidence of HT at this time point. The training cohort consisted of 733 Danish patients of whom 364 were consolidated with MR; 369 were not. Patients receiving MR more often had advanced clinical stage (90% vs 78%), high Follicular Lymphoma International Prognostic Index (FLIPI) score (64% vs 55%), and bone marrow infiltration (49% vs 40%). Those consolidated with MR had an improved 5-year overall survival (OS; 89% vs 81%; P = .001) and progression-free survival (PFS; 72% vs 60%; P < .001). In the training cohort, MR was associated with a reduction of HT risk (P = .049). Analyses of an independent validation cohort of 190 Finnish patients confirmed the favorable impact of MR on 5-year OS (89% vs 81%; P = .046) and PFS (70% vs 57%; P = .005) but did not find a reduced risk of HT. The present population-based data suggest that the outcome of patients with FL has improved after consolidation of R-chemo with MR.
Introduction
In western countries, follicular lymphoma (FL) is the second most common lymphoma entity after diffuse large B-cell lymphoma (DLBCL).1-3 FL is characterized by an indolent and chronic clinical course and apart from truly localized low burden disease, it is still regarded as an incurable condition with a natural history of recurrent relapses and, in a fraction of cases, transformation to an aggressive histology (histological transformation [HT]).4-7 When it occurs, HT is often associated with an unfavorable prognosis.8-12
Some FL patients are initially managed with an expectant approach without immediate need for antineoplastic treatment. For those where a therapeutic intervention is indicated (eg, high tumor burden, systemic symptoms, etc), the addition of the CD20 antibody rituximab to chemotherapy (R-chemo) has had a favorable impact on the time to progression, survival, and possibly also transformation rates.7,13-18 However, which induction regimen should be preferred is still debated. The most commonly used chemoimmunotherapy regimens are combinations of rituximab with either: cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP); cyclophosphamide, vincristine, and prednisone (R-CVP); or bendamustine (R-Benda).16,17,19
During the last decade, maintenance with rituximab (MR) after initial chemoimmunotherapy has been introduced with the hopes of further improving outcome for patients with FL. Several prospective and retrospective studies have demonstrated a beneficial impact of MR on outcome.20-28 This has been particularly evident with regard to progression-free survival (PFS), as shown by large randomized trials such as the Primary Rituximab and Maintenance (PRIMA)22 and Eastern Cooperative Oncology Group (ECOG) 149627 performed in the frontline setting. On the other hand, whether MR induces a significant improvement of overall survival (OS) and a reduction of the HT risk is still questioned.29-31
In the present Nordic collaborative study looking at population-based cohorts from Denmark and Finland, we investigated the effect of up-front MR on outcome and HT risk in FL patients managed with or without an intention-to-treat MR strategy.
Patients and methods
Training cohort
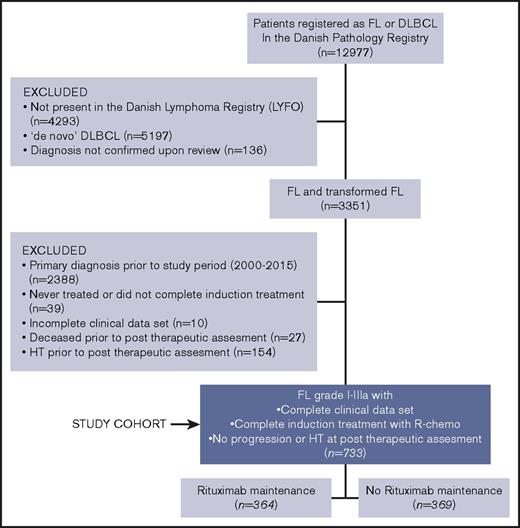
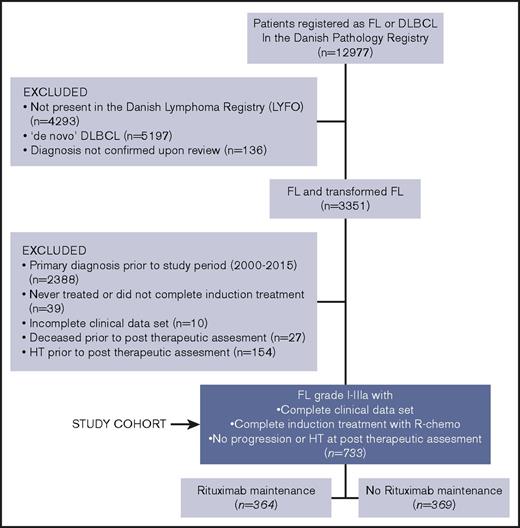
Patients diagnosed with histologically verified FL or DLBCL from 1990 to 2015 were identified through the National Danish Pathology Registry.32,33 Pathology reports were reviewed for patients diagnosed with both FL and DLBCL during the study period. HT was defined as a biopsy proven FL grade 1-3A followed by a FL grade 3B or a DLBCL histologically ascertained through a later biopsy. Subsequently, the identified patient cohort was cross-linked with the prospectively collected data of the Danish Lymphoma Registry (LYFO)34,35 and only patients with a full set of evaluable data covering pretherapeutic clinicopathological features, treatment parameters, and outcome end points at baseline and follow-up were included. From this population, patients were eligible to enter the final training cohort if meeting the following criteria: (1) FL diagnosis within the time period 2000-2015, (2) completed first-line induction treatment with R-chemo, (3) alive and with no evidence of relapse/progression nor HT according to an assessment performed at least 2 months after conclusion of the primary induction treatment.
Validation cohort
For validation, an independent population-based series of 190 patients with grade 1-3A FL treated at the Helsinki University Hospital Comprehensive Cancer Center between 2005 and 2015 were used. The patients were identified according to criteria corresponding to those used for the identification and end-point evaluation of the training cohort. This study was approved by the Danish Data Protection Agency and the National Committee on Health Research Ethics (no. 1-10-72-276-13) and done in accordance with the Declaration of Helsinki.
Rituximab maintenance
All patients completed a full course of R-chemo as first-line induction treatment. The decision to apply treatment-free follow-up or start MR was taken on a case-by-case basis at the discretion of the treating physician, also guided by national and international recommendations. For both the training and the validation cohort, MR consisted of 375 mg/m2 IV or subcutaneously given every second month for 2 years.
Response criteria
Pretherapeutic staging procedures were performed according to local guidelines and included diagnostic imaging with computed tomography or positron emission tomography–computed tomography scans, bone marrow biopsy (only repeated in patients with lymphoma infiltration at baseline), and ad hoc investigations in case of specific organ involvements. Treatment response was assessed according to the 1999 International Working Group criteria.36
Statistical methods
Patient characteristics were compared using the Fisher's exact or Student t test. OS was defined from the date of initiation of first-line induction treatment to the date of death by any cause or censoring; PFS was calculated from the date of initiation of first-line induction treatment to the date of progression/relapse or censoring. Time to transformation (TTT) and transformation-free survival (TFS) were calculated from the date of initiation of first-line induction treatment to the date of biopsy proven HT. All time-related end points were estimated by the Kaplan-Meier method and compared by the log-rank test if the assumption of proportional hazards was fulfilled; otherwise, a pseudo value approach for comparing survival functions at a fixed time point was used.37,38 Factors of potential clinical relevance were tested in a multivariate analysis using a either a Cox regression or a pseudo value approach.37,39 Risk estimates were expressed as hazard ratios (HRs) or risk differences (RDs) with 95% confidence intervals (CIs) at 5 years from first-line induction treatment. The risk of HT was evaluated in a competing risk model with death as a competing event, presented as cumulative incidence functions and compared using the model described by Pepe and Mori.40-42 HT-associated risk estimates at fixed time points were calculated using the pseudo values approach and expressed as cumulative incidence proportions (CIPs) and relative risk (RR) with 95% CIs at 5 years.40 All statistical analyses were performed using STATA (version IC 14; StataCorp, College Station, TX) or SPSS 22.0 (IBM, Armonk, NY).
Results
Training cohort
The training cohort consisted of 733 patients. All patients had completed their first-line rituximab-containing induction chemotherapy. Of the 733 patients, 364 received MR, whereas 369 did not. An algorithm describing how the training cohort was identified is shown in Figure 1. Pretherapeutic clinicopathological features and therapeutic background data of the training cohort are summarized in Table 1. The 2 treatment groups (no-MR vs MR) were comparable in terms of sex and age distribution, performance status and lactate dehydrogenase elevation. Conversely, patients receiving MR had more often disseminated disease (clinical stage III-IV; P < .001), a high risk profile (Follicular Lymphoma International Prognostic Index [FLIPI] intermediate/high; P < .001), multiple node involvement (P < .001), and bone marrow infiltration (P = .016). Of the 733 patients treated with rituximab-induction therapy, 286 (39%) received a doxorubicin-containing regimen (R-CHOP/CHOP-like) whereas 447 (61%) did not. Among the latter group, 347 (47%) were treated with R-CVP/CVP-like, 69 (9%) with R-Benda and 31 (4%) with a variety of other chemoimmunotherapy regimens. Doxorubicin-containing regimens were administered more often in patients that did not receive MR than in those who did (47% vs 31%; P < .001).
Outcome in the training cohort
The median follow-up was 4.7 years (0.2-14.0 years) for the entire cohort, 3.8 years (0.6-11.9 years), for patients consolidated with MR, and 5.7 years (0.2-14 years) for the patients who did not receive MR. The shorter median follow-up for MR patients was associated with a less frequent use of MR in the beginning of the study period.
Despite a more adverse risk profile, patients consolidated with MR had a significantly improved 5-year OS (89% vs 81%; P = .001), PFS (72% vs 60%; P < .001), and TFS (84% vs 76%; P = .002) (Figure 2; Table 2). This was true for both sexes (data not shown). Among patients with grade 3A histology, MR was administered to 40.2% (n = 72), whereas 59.8% (n = 107) did not receive it. In terms of impact, the PFS and OS values for no MR vs MR among grade 3A patients were in favor of MR: (1) PFS 62% (95% CI, 51-71) vs 73% (95% CI, 57-84) (P = .043) and (2) OS 80% (95% CI, 70-87) vs 90% (95% CI, 73-96) (P = .039), respectively.
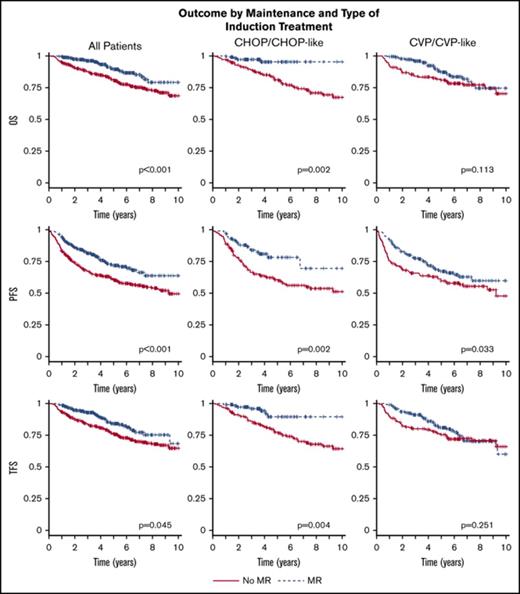
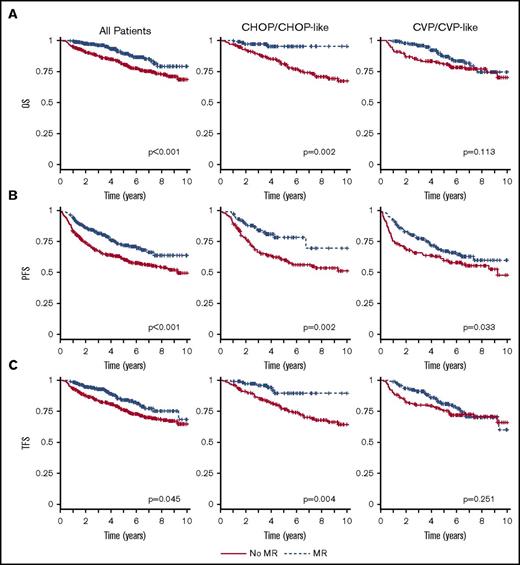
Outcome by maintenance and type of induction treatment. OS (A), PFS (B), and TFS (C) by maintenance and type of induction treatment (training cohort). Time calculated from initiation of first-line induction treatment.
Outcome by maintenance and type of induction treatment. OS (A), PFS (B), and TFS (C) by maintenance and type of induction treatment (training cohort). Time calculated from initiation of first-line induction treatment.
When considering different induction therapies, no significant effect of MR consolidation on either OS (87% vs 81%; P = .161), PFS (67% vs 60%; P = .183), or TFS (81% vs 76%; P = .260) was found for patients treated with R-CVP/CVP-like as induction therapy (Figure 2; Table 2). On the other hand, the patients treated with R-CHOP/CHOP-like had a favorable effect of MR on all outcome parameters, that is, OS (93% vs 81%; P = .003), PFS (77% vs 60%; P = .004), and TFS (88% vs 77%; P = .021). In the training cohort, the number of patients receiving R-Benda was low and no difference in outcome was detected between no MR and MR, probably due to insufficient statistical power.
Of the 733 patients representing the entire training cohort, 155 relapsed and 118 died. Upon relapse after MR or observation, 128 received salvage treatment consisting of R-chemotherapy (n = 105), rituximab only (n = 15), or chemotherapy only (n = 8). Overall, the fraction of patients receiving rituximab at first relapse was 80% in both the no MR and MR group. Twelve deaths (3%) were registered to have occurred among MR patients within a 2.5-year period from start of MR. Of these deaths, 2 (17%) were reported to be treatment-related.
In a multivariable adjusted analysis performed on the entire training cohort and adjusted for selected prognostic factors (FLIPI, use of anthracycline in the induction regimen, grade 3A histology, bulky disease, sex), MR significantly improved OS (RD, 8%; P = .019), PFS (RD, 15%; P < .001), and TFS (RD, 10%; P = .007) (Table 3). A high FLIPI score also predicted adverse outcome in term of OS (RD, −11%; P = .014) and PFS (RD, −14%; P = .024), but not TFS (RD, −8%; P = .135). In the subset of patients treated with doxorubicin-containing induction, MR still significantly improved OS (RD, 10%; P = .049), PFS (RD, 23%; P < .001) and TFS (RD, 12%; P = .028) after multivariate analysis. The same was not the case for FL histological grade 3A. In patients treated without doxorubicin only a high FLIPI risk score was able to predict outcome, whereas MR treatment was uninfluential (Table 3).
Impact of MR on risk of HT and TTT
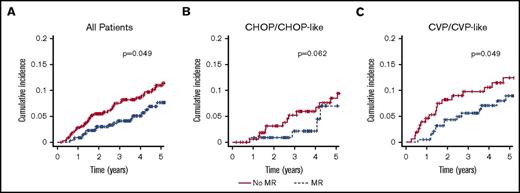
Among the 733 patients in the training cohort, 60 transformed to an aggressive histology. Of these, 54 (90%) transformed to DLBCL and 6 (10%) to FL grade 3B. There was no difference in terms of transformation histology between no MR and MR patients. An overall reduction in the risk of transformation was observed for patients treated with MR within the training cohort (P = .049; Figure 3). However when assessing the estimates at fixed time points this advantage was not significant (CIP at 5 years: 2.7 without MR vs 0.8 with MR; RR, 0.30; P = .069; Table 4). In terms of HT, the benefit of MR seemed more evident in patients who did not receive a doxorubicin-containing induction (Table 4; Figure 3), though the frequency of HT in CHOP/CHOP-like treated patients was overall low (6%; Table 4).
Cumulative incidence of HT by maintenance and type of induction treatment (training cohort). All patients (A), CHOP/CHOP-like (B), and CVP/CVP-like (C). Time calculated from initiation of first-line induction treatment.
Cumulative incidence of HT by maintenance and type of induction treatment (training cohort). All patients (A), CHOP/CHOP-like (B), and CVP/CVP-like (C). Time calculated from initiation of first-line induction treatment.
TTT was significantly shorter in patients that did neither receive doxorubicin-containing induction nor MR (2.6 years vs 1.3 years; P = .022). In these patients, the addition of MR improved TTT values up to a level comparable to that of R-CHOP/CHOP-like treated patients (Table 4). Interestingly, once histologically transformed, patients did not have different outcome regardless of prior MR or not (data not shown).
Validation cohort
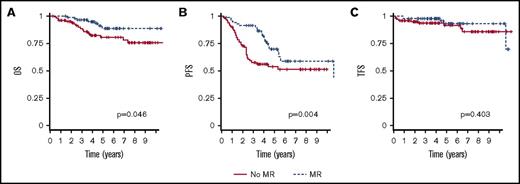
To validate the impact of MR on outcome, we analyzed an independent Finnish population-based cohort of 190 FL patients treated with R-chemo. Of them, 87 received MR and 103 did not. The pretherapeutic clinicopathological features and therapeutic background data of the validation cohort are listed in Table 1. Apart from B symptoms, which were more frequent in the group of patients receiving MR, baseline characteristics were equally distributed between the 2 treatment groups (no MR vs MR). However, R-CHOP was administered more often in patients that received MR than in those who did not (74% vs 49%; P = .001).The clinical outcomes according to treatment groups are shown in Table 2 and Figure 4. The patients consolidated with MR had a significantly better 5-year OS (89% vs 81%; P = .046) and PFS (70% vs 54%; P = .004), as compared with the patients who did not receive MR (Table 2; Figure 4). Although the risk of progression was reduced in the patients who received MR (HR, 0.487; 95% CI, 0.290-0.819; P = .007), no significant impact on the risk of HT was seen. In a multivariate analysis adjusted for FLIPI risk groups, and histological grade, a favorable prognostic impact of MR on PFS (HR, 0.470; 95% CI, 0.278-0.795; P = .005), and OS (HR, 0.406; 95% CI, 0.166-0.996; P = .049) was retained.
OS, PFS, and TFS by maintenance and type of induction treatment (validation cohort). OS (A), PFS (B), and TFS (C). Time calculated from initiation of first-line induction treatment.
OS, PFS, and TFS by maintenance and type of induction treatment (validation cohort). OS (A), PFS (B), and TFS (C). Time calculated from initiation of first-line induction treatment.
Discussion
In the present analysis of prospectively collected data from 2 large independent cohorts of FL patients, we found an outcome advantage for patients receiving up-front MR following R-chemo induction. This advantage was not limited to PFS, but it also included OS in both the training and the validation cohort.
A number of previous prospective and retrospective studies have also demonstrated a beneficial impact of MR on outcome.21,22,24-28 This has been particularly evident with regard to PFS. The results of the large randomized PRIMA trial comparing MR with observation without further therapy following R-chemo induction, showed an improved complete remission rate and PFS, but not OS in MR treated patients.22 Another trial testing MR after R-chemo induction in the front-line setting was performed in elderly (>60 years) FL patients treated with rituximab in combination with fludarabine, mitoxantrone and dexamethasone and followed by a short MR schedule of 4 bimonthly courses of rituximab. No PFS or OS advantage was found following MR as compared with observation only.28 On the other hand, the ECOG 1496 trial demonstrated a superior PFS in MR-treated patients after frontline induction with R-void CVP given every third week for a total of 6 courses.27 No concomitant OS prolongation was seen.
Several of the above-mentioned studies were included in a meta-analysis of individual patient data from 2315 FL patients enrolled in 7 randomized controlled trials of which 5 were conducted in the up-front setting.43 This analysis, demonstrated an OS benefit of MR compared with no MR which was consistent for relapsed/refractory disease but more questionable in the up-front setting after rituximab-containing induction.
Retrospective registry-based reports have shown MR-associated outcome advantages with variable end points, such as PFS,24,26,44 OS24,25,45 and time-to-next-treatment.26 Other studies have not confirmed these findings, identifying at best a trend toward better OS.26,46 Although, the general impression is that of a favorable impact of MR on outcome, the differences observed among these registry-based studies are probably due to heterogeneity in, for example, definition of target populations (eg, age, local vs disseminated disease, etc), choice of end points, length of follow-up, event verification, etc. Also in our study, 1 of the major limitations was the imbalance between parameters such as FLIPI score, FL histological grade 3A, response status, and use of anthracyclines in the induction regimen, as this may complicate the interpretation of a possible MR impact on outcome. Therefore, we performed a multivariable analysis (Table 3) with the specific intent to adjust for at least some of these imbalances. The result of this analysis showed that the favorable impact of MR on outcome was retained in both cohorts.
Another important consideration when evaluating the clinical impact of MR is the type of induction therapy used to achieve remission. In fact, and maybe somehow surprisingly, the benefit of receiving MR was greatest in the subset of patients treated with R-CHOP/CHOP-like induction (see Table 2 and Figure 2). In these patients, significantly better PFS, OS and TFS values were observed. The same was not the case for patients treated with MR after R-CVP/CVP-like regimens. This difference between doxorubicin-containing and doxorubicin-void induction persisted also after multivariable adjustment for anthracycline-containing regimens.
On the other hand, 2 recent phase 3 trials have shown no advantage for R-anthracycline-based regimens compared with R-Benda in the frontline setting of indolent lymphomas, including FL.19,47 However, none of these trials addresses the question of subsequent MR consolidation and their follow-up is still early.
A similar observation of improved PFS and OS after R-CHOP induction followed by MR was reported by 2 retrospective registry analyses, 1 by Janikova et al from the Czech Lymphoma Study Group25 and 1 by Cheah et al from the MD Anderson Cancer Center.24 Compared with these reports, the present study has 2 important advantages: (1) a markedly larger cohort size (twofold to threefold) and (2) the presence of a validation cohort.
The fact that a number of prospective and retrospective studies seem to report an advantage in PFS rather than OS for FL patients treated with MR after front line induction may reflect the prolonged OS of FL patients in the rituximab-era. In the light of a lower number of events, there will be an inevitable need of large study populations and extensive length of follow-up in order to demonstrate significant OS differences. This need may justify, despite of their inherent limitations, a role for large population-based retrospective analyses, such as the present one.
With regard to HT, a trend toward a reduction of HT risk was observed in the training cohort, but not confirmed in the validation set. The role of MR on the risk of HT in FL patients has been investigated in previous studies. In the recent update of the PRIMA trial, no significant impact of MR on HT occurrence was observed. However, the trial was not originally designed and powered to address this end point and histological verification at disease progression was performed in only a limited number of patients resulting in a possible underestimation of HT frequency.29 Recently, an analysis of prospectively collected registry data from the National LymphoCare Study demonstrated a significant impact of MR on the frequency of HT with a reduction from 13% to 9.2% (HR, 0.67; 95% CI, 0.46-0.97) compared with patients that did not receive MR.30 Also, a retrospective population-based analysis from the British Columbia Cancer Center suggested that the introduction of chemoimmunotherapy reduced the incidence of HT and that this effect was particularly evident in those patients that received MR (P = .003).31
The maintenance modality has recently also been adopted in the context of novel CD20 targeting antibodies.48,49 The recent GALLIUM study randomized rituximab vs obinutuzumab maintenance in patients with previously untreated advanced- stage FL and showed a PFS, but not OS, benefit in favor of obinutuzumab.48 The trial design did not include an “observation-only” cohort and results are therefore difficult to interpret with regard to the MR versus no MR debate. However, testing maintenance with novel second-generation anti-CD20 antibodies will most certainly be helpful in further clarifying the role of antibody maintenance for improving OS in FL patients.
In conclusion, the role of MR as part of the first-line standard of care strategy in treatment requiring FL is still debated. The present study adds to the existing data by showing that, in 2 independent cohorts of FL patients, the use of MR leads to an improvement of not only PFS, but also OS.
Acknowledgments
The authors thank all doctors and hematological departments who have contributed to collection of data to the Danish Lymphoma Registry (LYFO).
This work was supported by Region Midtjyllands Forskningsfond, Aarhus University Research Foundation, The Karen Elise Jensen Foundation, Helsinki University Hospital, Juselius Foundation, Finnish Cancer Societies, and Academy of Finland. M.R.C. was supported by the Program for Clinical Research Infrastructure (PROCRIN), Medical Biometrics, Department of Clinical Epidemiology, Aarhus University Hospital, Aarhus, Denmark.
Authorship
Contribution: C.M. and F.A.d’A. were responsible for study conception and design; C.M. and T.L.P. revised all pathology reports; C.M., M.R.C., A.M.P., S.L., and F.A.d’A. planned and performed data analysis; C.M., M.L., S.L., and F.A.d’A. drafted the manuscript; and all authors contributed to collection, assembly, and interpretation of data, and took part in revision and final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Francesco A. d’Amore, Department of Hematology, Aarhus University Hospital, Tage Hansens Gade 2, 8000 Aarhus, Denmark; e-mail: frandamo@rm.dk.