Background
The care of patients with lymphoma relies heavily on accurate tissue diagnosis and classification.
In sub-Saharan Africa (SSA), where the lymphoma burden is increasing due to population growth, aging, and continued epidemic levels of HIV infection, pathology services are extremely limited.
Cancer registry data show that only 18% of all cancers diagnosed in Malawi were pathologically confirmed.
Diagnostic pathology services were not previously available in Lilongwe, the capital city of Malawi.
In 2011, through a joint effort between the Malawi Ministry of Health, the Kamuzu Central Hospital (KCH), and the University of North Carolina (UNC), a pathology laboratory was established in Lilongwe at KCH to support a prospective lymphoma clinical trial.
The laboratory provides routine diagnostic service, clinical trials, and a research program that has attracted Malawian pathologists and technologists to a center that lacked any pathology services 6 years prior.
Methods
The initial equipment and laboratory start-up costs were ∼$200 000 US dollars, including a digital microscopy system and infrastructure for basic tissue processing and histology.
Current equipment and available immunohistochemical stains (IHC) are listed in Table 1.
All cases are reviewed by local pathologists, and difficult cases as well as diagnostic specimens from all patients enrolled in the ongoing KCH Lymphoma Study are reviewed at a weekly telepathology conference (basic workflow: Figure 1)
Telepathology conferences are attended by Malawian clinicians, Malawian pathologists, and their counterparts in the United States.
Diagnoses are issued by local pathologists.
To ensure quality control and facilitate research studies, tissue blocks and glass cytology slides for all KCH Lymphoma Study patients are sent to collaborators at UNC quarterly.
Histology cases are further characterized by a broader panel of IHC and in situ hybridization stains, and a final diagnosis is rendered.
Diagnostic agreement was assessed using a 4-tier scoring system to quantify concordance between real-time diagnoses rendered at weekly telepathology conferences and final diagnoses rendered in the United States.
Results
By 2016, the laboratory processed 5611 histology and 1998 cytology cases, the majority of these interpreted by 1 of 2 local pathologists without international consultation or additional stains (Figure 2).
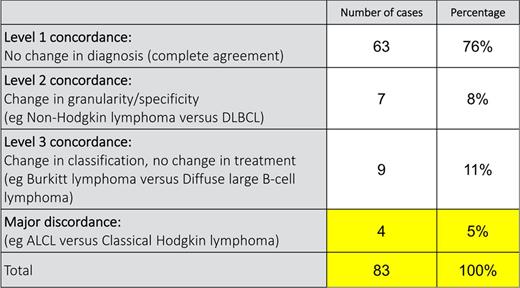
Although the expanded immunophenotyping offered in the United States permits more granular classification of some lymphomas, an initial diagnosis made in Malawi led to appropriate treatment in 95% of cases (n = 79 of the first 83 diagnosed lymphomas) (Table 2).
Concordance mirrors that seen in the United States after central review for cancer diagnosis.
Pathologists and the laboratory have passed the accreditation exam for participation in the AMC-068 HIV lymphoma trial.
Future directions
Expand panel of IHC stains.
Increase automation of laboratory workflow to accommodate increasing clinical volume.
Continue mentoring for Malawian pathologists and technicians.
Implement validated molecular diagnostic assays.
Support expansion of the National Institutes of Health research initiative.
Develop regional collaborations with other African pathology centers of excellence.
Conclusions
Hematologic disorders, including lymphoma, in SSA are a major cause of morbidity and mortality.
Developing high-quality pathology services for routine care, clinical trials, and basic research in resource-limited settings is feasible.
Improvement of the regional capacity to accurately classify tumors is paramount to understanding true disease epidemiology and to establish effective treatment strategies.
Authorship
Conflict-of-interest disclosure: No competing financial interests declared.
Correspondence: Y. Fedoriw, Department of Pathology and Laboratory Medicine and Lineberger Comprehensive Cancer Center, University of North Carolina, Chapel Hill, NC; e-mail: Yuri.Fedoriw@unchealth.unc.edu.