Key Points
Physician-assessed clinical responses and immunohistochemical changes were seen in association with sonidegib therapy for cGVHD.
Sonidegib therapy was limited by ongoing cGVHD symptoms and adverse events not attributed to treatment.
Abstract
Hedgehog signaling plays a key role in tissue fibrosis, the pathological hallmark of chronic graft-versus-host disease (cGVHD). We conducted a phase 1 trial of sonidegib, a selective antagonist of the hedgehog coreceptor Smoothened, for the treatment of steroid-refractory cGVHD. After a 3+3 study design, sonidegib was administered for up to 12 cycles of 28 days each, using 3 doses: 200 mg/day (dose level 1), 400 mg/day (dose level 2), and 600 mg/day (dose level 3). Seventeen patients were enrolled. The median number of cycles completed was 6 (range, 0-12). There was only 1 dose-limiting toxicity (cohort 2, grade 3 creatine phosphokinase increase) observed. Immunohistochemical evaluation of skin biopsies revealed decreased protein expression of hedgehog signaling pathway molecules with sonidegib therapy. Clinically, 8 patients (47%) had a partial response in skin or sclerodermatous disease, 6 patients had no response, and 3 were not evaluable. Clinical responses were assessed by treating physicians and not by National Institutes of Health criteria. Overall, patients reported worsening of quality of life, which was more severe in clinical nonresponders. Accrual was terminated early as a result of the cumulative toxicity burden not attributed to sonidegib and patient decisions to stop taking sonidegib. We believe hedgehog signaling inhibition warrants further investigation in patients with cGVHD because of the association with clinical responses and immunohistochemical changes. This trial was registered at www.clinicaltrials.gov as #NCT02086513.
Introduction
Chronic graft-versus-host disease (cGVHD) is a leading cause of long-term morbidity after allogeneic hematopoietic cell transplantation, with a 2-year cumulative incidence of cGVHD requiring systemic treatment of 30% to 40%.1-3 Although corticosteroids remain the mainstay of systemic therapy, treatment is often prolonged, and 50% to 60% of patients require additional agents within 2 years.4,5 For years, there has been no standard second-line therapy for cGVHD. Ibrutinib recently became the first therapy approved by the US Food and Drug Administration for cGVHD, and studies investigating agents that target novel pathways are still of utmost importance. Hedgehog signaling is active in human and murine sclerodermatous cGVHD.6 Downstream actions of this cascade result in accumulation of the transcription factors Gli-1 and Gli-2, particularly in fibroblasts, which may stimulate the release of collagen and lead to the pathologic fibrosis seen in cGVHD. This phase 1 study examined the safety of sonidegib, a selective small molecule antagonist of the hedgehog coreceptor Smoothened (Smo), in the treatment of steroid-refractory cGVHD.
Methods
This study was approved by the institutional review board at the Dana Farber Harvard Cancer Center. Informed consent was obtained from all patients. This trial was registered at ClinicalTrials.gov (NCT02086513). Patients were 18 years of age or older and had a diagnosis of steroid-refractory classic cutaneous, myofascial, or sclerodermatous cGVHD (± other organ involvement). Steroid-refractory cGVHD was defined as requiring 0.25 mg/kg per day or more of prednisone (or equivalent), disease that flared/progressed on corticosteroid taper, or patients with contraindication for systemic corticosteroid therapy. Patients may have received any number of prior cGVHD therapies and could not have active disease relapse.
Sonidegib was given daily in 28-day cycles by continuous dosing. A traditional 3+3 study design was used with the primary endpoint to determine the maximum tolerated dose of sonidegib in the treatment of steroid-refractory cGVHD. Three escalating doses of sonidegib were used: 200 mg daily (dose level 1), 400 mg daily (dose level 2), and 600 mg daily (dose level 3). The period of dose-limiting toxicity (DLT) evaluation was 2 cycles of 28 days (56 days total). Patients were taken off study if there was clear cGVHD progression at the start of cycle 3 or any subsequent point. Patients were considered not evaluable for the determination of maximum tolerated dose if they were removed from the study for reasons unrelated to therapy within 56 days of starting treatment. DLT was defined as drug-related grade 3 or 4 adverse event (AE) that occurred during the first 2 cycles of treatment. Nausea, vomiting, and diarrhea that could be controlled with routine supportive measures along with alopecia were not considered a DLT. Hematological DLTs only included grade 4 neutropenia or thrombocytopenia. No taper of concurrent systemic immunosuppression was allowed until the start of cycle 2. Addition of any additional systemic immunosuppression or initiation of extracorporeal photopheresis for progression of cutaneous, myofascial, or sclerodermatous cGVHD during the treatment period mandated removal from the trial.
Results
Seventeen patients were treated, including 6 receiving dose level 1 (200 mg), 6 receiving dose level 2 (400 mg), and 5 receiving dose level 3 (600 mg). Baseline patient characteristics are summarized in Table 1. Three patients were not evaluable for DLTs, as they withdrew from the study because of bleeding esophageal varices, cardiac arrest, and patient decision. There was only 1 formal DLT (cohort 2, grade 3 CPK increase) observed. The maximum tolerated dose was not reached. All 17 patients (100%) experienced grade 3 or 4 AEs unrelated to sonidegib. The most common grade 3 or 4 AEs unrelated to sonidegib were arthralgia (n = 3), abdominal pain (n = 3), myalgia (n = 3), back pain (n = 2), headache (n = 2), and hypercalcemia (n = 2). Additional grade 3 or 4 AEs observed (single events) included anemia, cardiac arrest, chest pain, congestive heart failure, diarrhea, hypertension, hypotension, port infection, skin discoloration, small bowel obstruction, and superior vena cava syndrome.
Three patients completed the planned 12 cycles of treatment. The median number of cycles completed was 6 (range, 0-12). Reasons for ending therapy included patient decision (n = 9), lack of response (n = 4), and death (n = 1). The 1 death was a result of bleeding esophageal varices that occurred during cycle 1 of therapy (not related to sonidegib). Overall, 8 patients (47%) had a partial clinical response limited to cutaneous or sclerodermatous disease, as judged by treating physicians; 6 patients (35%) had no response, and 3 were not evaluable. There were no complete responses observed. Fifteen patients were taking corticosteroids at the time of study enrollment, with a median daily dose of 15 mg (range, 5-30 mg). At the time of ending sonidegib therapy, 6 patients (40%) were able to decrease their baseline prednisone dose by at least 25%. No patients required a higher dose of prednisone while in the study. The study was closed prematurely because of the significant cumulative toxicity experienced by patients without any complete responses, resulting in increasing patient decisions to stop taking sonidegib.
Patient-reported cGVHD symptom severity and quality of life were assessed using the Chronic GVHD Symptom Scale and the Functional Assessment of Cancer Therapy-General, respectively. Assessments were collected at baseline, cycle 4, cycle 7, and 12 months after initiation of sonidegib. Survey results were analyzed as a comparison of baseline evaluation and last evaluation obtained and were not adjusted for time on study. The change in mean cGVHD symptom score over time did not meet statistical significance for clinical nonresponders (26 to 33.2; P = .09), nor for clinical responders (34.4 to 30.14; P = .23). In addition, there was a significant difference when comparing mean change in cGVHD symptom scores between clinical responders and nonresponders (−4.28 vs 7.2; P = .04). Moreover, clinical nonresponders reported a significant decline in their quality of life over time (87 to 72.5; P = .04), whereas reported changes for responders was less prominent (76.16 to 71.3; P = .55). However, when comparing the mean change in Functional Assessment of Cancer Therapy-General scores between responders and nonresponders, there was no significant difference (−4.86 vs −14.5; P = .36).
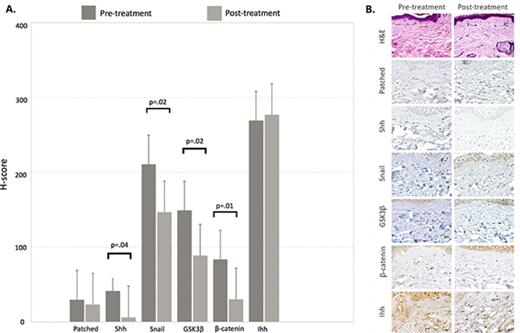
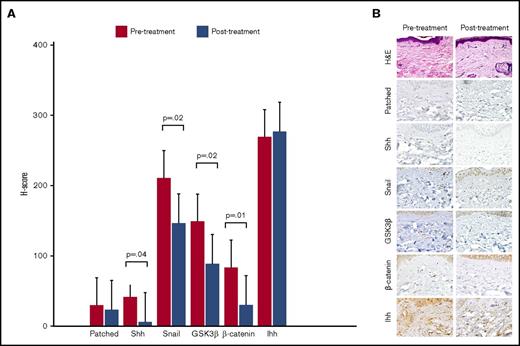
Punch biopsies were obtained from the same site of cGVHD-affected skin before sonidegib therapy and at the beginning of cycle 2. Immunohistochemical analysis using anti-human antibodies directed against hedgehog signaling pathway molecules was performed and H-score calculated for each molecule pre- and posttreatment. The dermatopathologists were blinded as to whether samples were pre- or posttreatment. For the 13 patients with posttreatment biopsies, sonidegib therapy was associated with a significant decrease in mean H-score for sonic hedgehog (Shh), Snail, GSK3-β, and β-catenin, respectively (Figure 1A). Immunohistochemical staining of skin biopsy from a sample patient with a clinical response is shown in Figure 1B.
Immunohistochemical evaluation of hedgehog signaling pathway molecules. (A) Mean H-scores for hedgehog signaling pathway molecules obtained from pre- and posttreatment skin biopsies from 13 evaluable patients. The H-score is calculated by multiplying the percentage of cells staining for a given molecule in a fixed field by the staining intensity (0 = none, 1 = weak, 2 = moderate, 3 = intense), with final scores ranging from 0 to 300. (B) Histochemical staining demonstrating a decrease in staining intensity for hedgehog pathway molecules in skin biopsies from a sample clinical responder. Hematoxylin and eosin (H&E) stain, original magnification ×600; all other stains, original magnification ×200. GSK3β, glycogen synthase kinase 3 β; Ihh, Indian hedgehog; Shh, sonic hedgehog.
Immunohistochemical evaluation of hedgehog signaling pathway molecules. (A) Mean H-scores for hedgehog signaling pathway molecules obtained from pre- and posttreatment skin biopsies from 13 evaluable patients. The H-score is calculated by multiplying the percentage of cells staining for a given molecule in a fixed field by the staining intensity (0 = none, 1 = weak, 2 = moderate, 3 = intense), with final scores ranging from 0 to 300. (B) Histochemical staining demonstrating a decrease in staining intensity for hedgehog pathway molecules in skin biopsies from a sample clinical responder. Hematoxylin and eosin (H&E) stain, original magnification ×600; all other stains, original magnification ×200. GSK3β, glycogen synthase kinase 3 β; Ihh, Indian hedgehog; Shh, sonic hedgehog.
Discussion
This phase 1 study represents the first prospective clinical evaluation of hedgehog signaling inhibition in the treatment of cGVHD. We found that certain patients may have benefited from sonidegib therapy, as they experienced clinical responses as judged by treating physicians. A significant cumulative toxicity burden was experienced by patients on this study and led to discontinuation of therapy. There is overlap between known AEs of sonidegib and cGVHD symptoms, such as abdominal pain, myalgias, and headaches. Most of these toxicities were not attributed to sonidegib, but the delineation of treatment-related symptoms from disease-related symptoms in patients with active steroid-refractory cGVHD remains challenging. Evaluation of skin biopsies showed decreased expression of hedgehog pathway signaling molecules after initiation of sonidegib. However, the decreased expression of Shh was surprising, as sonidegib would not be expected to alter Shh protein expression according to the proposed mechanism of action.6 The immunohistochemical analysis, in the context of the clinical response, may indicate that sonidegib therapy results in improvement in sclerodermatous cGVHD via hedgehog signaling inhibition. Future investigations into this pathway would be possible, as several components of the hedgehog signaling, including Shh, Smo, and Gli-1/2, are viable therapeutic targets with inhibitors either in development or in clinical trials.7 A randomized clinical trial with more extensive correlative analyses, such as hedgehog target gene expression in addition to protein expression, would be required to more definitively characterize the clinical role of hedgehog pathway inhibition in cGVHD.
We believe this trial highlights difficulties with early-phase clinical trials in this population. Patients with steroid-refractory cGVHD have often received multiple lines of immunosuppressive therapy, which increases the likelihood for treatment-related morbidity. Furthermore, it remains difficult to achieve complete responses in steroid-refractory cGVHD, as the majority of responses are partial in nature, leaving patients with ongoing disease-related symptoms and the likelihood of pursuing additional therapies. To enhance clinical response, combination therapies could potentially be considered, albeit with an increased risk for additional toxicity.
The therapeutic landscape for cGVHD is changing, highlighted by the recent US Food and Drug Administration approval of ibrutinib for cGVHD after failure of 1 or more treatments. Novel approaches to cGVHD therapy are shifting away from systemic immunosuppression and moving toward targeting pathways involved in fibrosis and inflammation, with the hope of eliciting disease response with fewer off-target effects. However, given the complex pathophysiology of cGVHD, the goal of developing well-tolerated therapeutics that achieve better clinical responses remains an immense challenge.
Acknowledgment
Novartis, Inc., provided LDE225 as well as clinical trial funding.
Authorship
Contribution: S.L. and Y.-B.C. designed the study; Z.D., R.M.N., A.E.-J., and Y.-B.C. analyzed and interpreted the data; Z.D. and Y.-B.C. wrote the manuscript; all authors reviewed and approved the paper; and Y.-B.C. had the final responsibility over the decision to submit for publication.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yi-Bin Chen, 55 Fruit St, Yawkey 9E-9052, Boston, MA 02114; e-mail: ychen6@partners.org.