Despite the increasing use of rituximab in the treatment of idiopathic thrombocytopenic purpura (ITP), there is a remarkably small amount of literature describing its efficacy and toxicity. The authors of this study reviewed all of the articles on this topic published between 1997 and 2004 and found only 19 reports that described five or more patients (313 total patients). Of these 19 reports, nine were published only in abstract form. The median duration of followup was only 9.5 months (range: two to 25 months). All of the publications were case reports, case series, or single-arm cohorts, and there were no randomized trials. The reported response rates were higher in studies that contained small numbers of patients, making definitive conclusions about the response rate difficult.
Pre-treatment platelet counts varied between 1,000 – 89,000/µl. Almost all of the patients had been previously treated with corticosteroids, and approximately half of the patients had failed a splenectomy. Patients were treated with four weekly doses of 375mg/m2 in 16 of the 19 studies, while the remainder did not state the dose, or varied either the dose or the duration of therapy. The overall response rate (platelet count >50,000) was 63 percent, and the complete response rate (platelet count >150,000) was 46 percent. The median duration of a response was 10.5 months (range: three to 20 months). Although the median time to a response was 5.5 weeks, it was curiously as short as two weeks in some series (more on this topic later). The majority of the treatment-induced toxicity was infusion-related serum sickness, or other allergic responses. Nine deaths were reported, and most of these events were hemorrhagic or due to an underlying comorbidity. Several patients died of infections, but not clearly related to treatment.
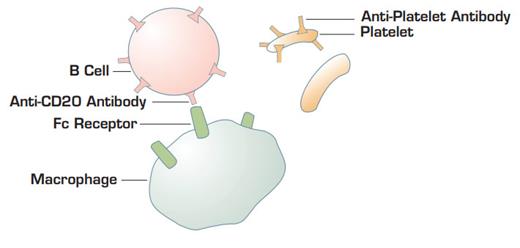
Proposed model by C.M. Bennett and colleagues. Anti-CD20 coating B cells bind to Fc gamma receptors on macrophages. This interaction blocks Fc receptors and retards the ability of macrophages to bind and endocytose immunoglobulin-coating platelets.
Proposed model by C.M. Bennett and colleagues. Anti-CD20 coating B cells bind to Fc gamma receptors on macrophages. This interaction blocks Fc receptors and retards the ability of macrophages to bind and endocytose immunoglobulin-coating platelets.
In Brief
Splenectomy has long been considered the gold standard of therapy for patients who require treatment for chronic ITP. It produces long-term response rates of 65-70 percent, and many of the remaining patients derive some benefit from the procedure. Although splenectomy has an impressive response rate, it is associated with approximately 1 percent operative mortality, as well as a lifelong increased risk of opportunistic infections. The alternative standard therapy is chronic immunosuppressive drug therapy.
The monoclonal antibody against CD20-positive B cells, rituximab, has received recent enthusiasm as an immunosuppressive agent for a wide variety of autoimmune diseases. It is clear that rituximab can induce remissions in ITP. Although, it has been difficult to estimate its true efficacy since the literature is biased by small case series touting high response rates. By restricting analysis to publications that report response rates based on more than five patients, Arnold and colleagues identified only 10 papers and nine abstracts that focused on rituximab efficacy in ITP. Their results place the response rate of rituximab in the same category, but probably not better than most other immunosuppressive agents. However, rituximab still does have the advantage of a relatively good safety profile.
Does this mean that rituximab is a reasonable alternative treatment for ITP patients who require chronic therapy? Although it is being used with increasing frequency as hematologists become more familiar with the drug, we really cannot state definitive efficacy rates or long-term toxicity data to our patients as we can for splenectomy treatment. It is also unclear how anti-B-cell therapy for ITP can sometimes produce a response within a few days. Circulating IgG has a half-life of approximately three weeks, so if rituximab's effect is to stop all additional immunoglobulin production, its response should not be apparent for several weeks to months. One possible explanation for the rapid effect is that rituximab-coated B cells may compete with immunoglobulin-coated platelets by the reticuloendothelial system1 . However, it is surprising that enough antibody-coated B cells are generated after rituximab therapy to mimick a "WinRho-like" effect. If this model for the rapid rituximab effect is correct, then one would predict that it would not be seen in post-splenenctomy patients. Thus far, this does not appear to be the case.
Although rituximab is clearly a useful agent in the treatment of ITP, the published data is currently a little thin. It would therefore be worthwhile and beneficial for both patients and physicians to study rituximab in a larger cohort. Only then will we be able to definitively understand the efficacy and toxicity of this therapy for ITP.
References
Competing Interests
Dr. Abrams indicated no relevant conflicts of interest.