Abstract
TP53-mutated myeloid disease is a constellation of abnormalities seen in both de novo and therapy-related acute myeloid leukemia and myelodysplastic syndrome. Historically, this group of disorders has had a poor prognosis. Newer treatment combinations allow patients to be treated with less toxicity. If response to induction therapy is achieved, fit and willing patients should be considered for allogeneic hematopoietic cell transplantation (HCT). The addition of allogeneic HCT to the treatment approach has modestly improved outcomes compared to chemotherapy alone, more so for those patients with disease control. Tailoring the conditioning regimen and maintenance therapy may improve outcomes in TP53 myeloid patients. In addition to chemotherapy, disease-modulating and immunological treatments continue to be studied to further improve outcomes.
Learning Objectives
Identify which patients may benefit from allogeneic HCT
Review the ways in which allogeneic HCT provides an immunological therapy for TP53-mutated disease
CLINICAL CASE
A 60-year-old, otherwise healthy gentleman presented with thrombocytopenia and mild anemia. After initial evaluation a bone marrow (BM) biopsy demonstrated a normocellular marrow with megaloblastic erythropoiesis, dyspoesis, and 5% blasts by flow cytometry, consistent with myelodysplastic syndrome with excess blasts (MDS-EB1). He had an abnormal karyotype: 44 X, −Y, del(1)(p13), −5, del(6)(q13q23), add(7)(p11.2), der(8)t(1;8)(p31;p23), −12, del(17)(p11.2p13), +22 [4]/46 XY [16]. Next-generation sequencing (NGS) resulted a single mutation of TP53 c.994-1G>A; variant allele frequency, 7%. Findings were consistent with high-grade MDS. He was treated on a randomized clinical trial of azacitidine (AZA) and eprenetapopt vs AZA, randomized to control azacitidine for 4 cycles. Pre-transplant BM noted a diploid karyotype in 19 of 20 metaphases with 1 residual complex karyotype. Myeloblasts were 1.5% by flow cytometry, and NGS was negative for mutations. What would your next step be?
Introduction
TP53-mutated myeloid disease includes a constellation of abnormalities involving the short arm of chromosome 17(p13), seen in both de novo and therapy-related acute myeloid leukemia (AML) and MDS. In both AML and MDS, the TP53 gene is associated with structural changes and complex chromosomal abnormalities, leading to limited response to routinely used therapeutic choices, often conferring a poor prognosis.1,2TP53 mutations produce resistance to both intensive chemotherapy (IC) and the body's immunological defenses, making this mutational entity inherently challenging to treat.3-5
Newer treatment combinations, as an alternative to IC, allow more patients to be treated with fewer complications and a lower toxicity burden. In both AML and MDS patients, the usage of hypomethylating agents (HMAs), azacitidine (AZA), or decitabine as up-front treatment has demonstrated responses comparable to IC, with significantly less toxicity.6,7 In treatment-naive patients with AML, HMA and venetoclax (VEN) therapy has produced complete remission (CR) rates ranging from 41 % to 47 %, with a median overall survival (OS) of 5 to 7 months.8,9 A recent systematic review and meta-analysis evaluated IC vs HMA-VEN vs HMAs alone in treatment-naive AML patients. Of the 12 studies that met inclusion criteria, IC was associated with the highest CR rate, 43 %, vs CR rates of 33 % for HMA-VEN and 13 % for HMA alone. The median OS was poor for all groups, approximately 6 months. The authors concluded that there is a significant need for improved treatments for this difficult population.10 To date there are no novel treatments or combinations that have significantly improved TP53 myeloid disease outcomes.8,11-13
CLINICAL CASE (continued)
After a discussion of treatment options, our patient decided to proceed to an allogeneic myeloablative hematopoietic cell transplant (HCT) from a matched unrelated donor. Post HCT, he was placed in a maintenance therapy clinical trial of eprenetapopt and AZA. He began 60 days post transplant and received 12 cycles of monthly therapy. A BM biopsy after cycle 10 showed CR with normal NGS and cytogenetics. A restaging BM biopsy 1 month after completion of maintenance therapy showed high-grade MDS with 15 % to 20 % blasts and abnormal complex cytogenetics consistent with progression of his disease. He received decitabine and VEN response and later a universal chimeric antigen receptor (CAR) T-cell treatment. He ultimately decided on hospice care after failing all attempts to attain disease control.
Allogeneic HCT considerations
The transplantation of healthy hematopoietic stem cells from an allogeneic donor (HCT) is a treatment option to improve outcomes for other high-risk malignancies. With the development of safer transplant-conditioning regimens, supportive care measures, and enhanced donor selection methodologies, HCT can now be offered to a wider population, with appropriate patient selection criteria.14 Given the known rigors of HCT (treatment-related morbidity and mortality, organ toxicity, infectious complications), careful patient selection is crucial to optimize outcomes.15,16
Multiple trials have demonstrated worse outcomes, including worse OS, increased rate of relapse, and higher nonrelapse mortality, in TP53 myeloid disease compared to those patients with non–TP53-associated mutations.17-19 A recent systematic review and meta-analysis of patients receiving allogeneic HCT for TP53-mutated disease demonstrated a 21% OS with a very high relapse rate of 58.9%. Despite these unfavorable results, the prospective utilization of HCT has shown benefits for patients with TP53 compared to conventional treatments.20
To directly address the question about the efficacy of a potentially curative approach to HCT in comparison to a more conservative nontransplant approach, the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) conducted a large, prospective, multicenter, biologic assignment trial (BMT CTN 1102) comparing MDS patients in a contemporaneous donor/no donor analysis. Specifically evaluating study participants with a TP53 mutation who underwent HCT, those patients had an improved 3-year OS of 23% compared with 7% of patients in the non-HCT arm (P = .04).21 This gives weight to the option to offer allogenic HCT in the treatment of this high-risk mutation.
Pretransplantation response
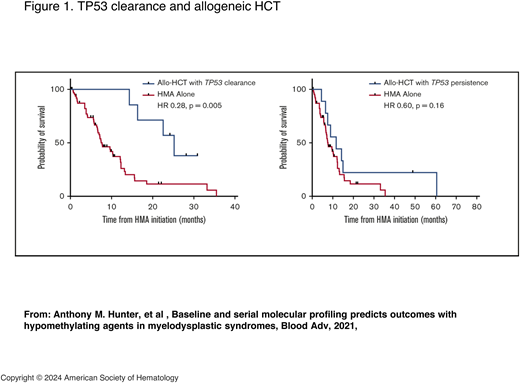
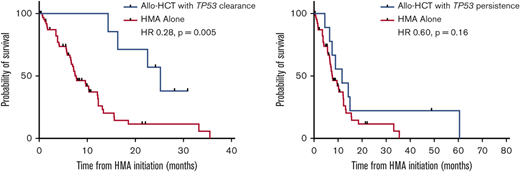
There is appropriate concern in taking patients with a TP53 mutation to allogeneic HCT, particularly if they have multihit TP53, TP53 associated with complex cytogenetics, or monoclonal karyotype or copy number loss of heterogeneity.1 However, this should be balanced with patients who respond both morphologically and particularly molecularly to induction therapy. Patients with clearance of the TP53 mutation have better outcomes than those patients who have persistently high variant allele frequency or measurable residual disease (MRD) at the time of transplant (Figure 1).22-24 Even with this more favorable selection of responding patients, allogeneic HCT should be presented with the sobering reality that relapses remain high, and survival is not guaranteed.
TP53 clearance and allogeneic HCT. HR, hazard ratio. Adapted with permission from Hunter et al.24
TP53 clearance and allogeneic HCT. HR, hazard ratio. Adapted with permission from Hunter et al.24
A recent study of patients with therapy-related MDS evaluated both the genetic and clinical factors impacting outcomes in this disease. TP53 was the most common abnormality seen, with up to 30 % of patients impacted. Therapy-related MDS patients were compared to patients with de novo disease and were found to have no statistically significant difference in survival between the 2 cohorts (49.9 % vs 53.9%; P = .61). The therapy-related group had an increased propensity for high-risk mutations. Only 5 out of 18 (28%) of the patients with TP53 mutations had multihit disease.16
To further understand the impact of treatment paradigms on AML outcomes, the COMMAND study, a multicenter, retrospective analysis, critically evaluated factors that predicted survival in TP53-mutated disease. Of the 370 patients with AML with TP53 mutation, 18 % (68 patients) had HCT. This large study took patients in first and second CR/CR with incomplete hematologic recovery to transplant with both myeloablative (57%) or reduced intensity (43%) conditioning. This analysis included patients with persistent disease at the time of transplant. The median OS was 24.5 months. In multivariate analysis, CR at day 100 and chronic graft-versus-host (GVHD) disease were factors for improved event-free survival and OS. The authors concluded that HCT offered the best opportunity to improve long-term outcomes in patients with TP53-mutated AML with the currently available treatment regimens.25
Conditioning regimen
Conditioning regimen may also have an impact on survival. Data from the BMT CTN 0901 trial demonstrated that myeloablative conditioning was preferable to those patients with AML and MDS with TP53 MRD at the time of HCT, producing a reduction in relapses and improved OS.22,23 The problem is that most of the patients with these myeloid disorders are older than 65 years and have significant comorbidities. In this setting a myeloablative approach is not feasible. These findings are contrary to a prior study in patients with MDS TP53 disease that showed no impact of a more intense conditioning regimen.18 The BMT CTN 0901 trial demonstrates the importance of tailoring the conditioning regimen for optimal outcomes.
Post-HCT maintenance
HCT is the quintessential adoptive immunological therapy. However, HCT alone is not enough to control TP53-mutated myeloid disease. Disease relapse remains the leading cause of HCT failure despite improvement in HCT treatment paradigms.26 Maintenance therapy may decrease the risk of relapse post HCT, but questions remain regarding the identification of ideal patient subsets, minimal residual disease testing, duration of treatment, and drug intervention. Recent trials involving several pharmacologic agents are presented below.
Azacitidine plus venetoclax
The transplant program at the Dana-Farber Cancer Institute performed a phase 1 trial assessing the safety of maintenance VEN and AZA in high-risk MDS/AML patients undergoing a reduced-intensity allogenic HCT.27 These patients were conditioned with VEN, busulfan, and fludarabine followed by HCT with tacrolimus and methotrexate GVHD prophylaxis. Of these patients, 96% had a positive MRD status at the time of transplant. Twenty-two patients received maintenance therapy with AZA and VEN at 400 mg orally for 14 of every 28 days for up to 1 year. The median OS was not reached. Among the 22 patients who received VEN or AZA post HCT, the 2-year OS was 67 %. This included 16 of 27 patients (59.3 %) positive for TP53. Nine patients relapsed, and 8 of those 9 had TP53, with 5 of those 8 being biallelic. Two of the 9 were able to go into CR with immune suppression withdrawal. Larger studies with this approach should be considered.
Eprenetapopt (APR-246) maintenance
Eprenetapopt, a first-in-class, small-molecule p53 reactivator, has been extensively studied in TP53-mutated disease. In the setting of induction therapy, the phase 3 clinical trial evaluating the safety and efficacy of eprenetapopt with AZA vs AZA alone in 154 patients with TP53-mutant MDS terminated early due to futility.28
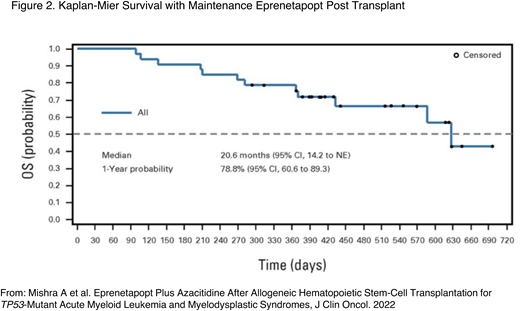
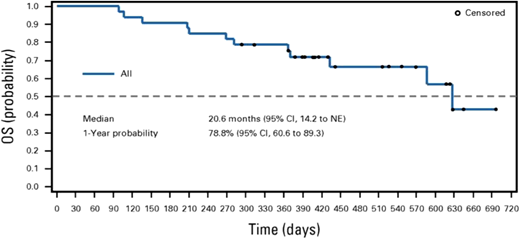
Prior to the negative outcome of the phase 3 trial, a phase 2 prospective multicenter, open-label trial of patients being treated with TP53-mutated myeloid disease evaluated the benefit of targeted maintenance therapy in the post-HCT setting. Of the 84 patients enrolled in the trial, 55 received an allogeneic HCT per the centers' standard of care. Eighty-three percent of the patients were TP53 MRD positive prior to transplant. Patients who were in morphological remission upon their posttransplant BM biopsy, had no evidence of GVHD, and had count recovery received the maintenance therapy. Thirty-three patients were treated with AZA for 5 days and eprenetapopt for 4 days in each 28-day cycle for 1 year or until disease progression. One-year relapse-free survival (RFS) and OS were 59.9% and 78.8% (Figure 2), respectively, meeting the studies' primary end point.29
Kaplan-Meier survival with maintenance eprenetapopt post transplant. NE, not evaluable. Adapted with permission from Mishra et al.29
Kaplan-Meier survival with maintenance eprenetapopt post transplant. NE, not evaluable. Adapted with permission from Mishra et al.29
The likelihood of eprenetapopt moving forward in the posttransplant setting is slim based on the negative phase 3 results. An oral p53 reactivator (APR-548) terminated its recently opened phase 1 trial (NCT 04638309). Thus far, minimal gains have been made with targeted molecules in posttransplant maintenance. Much more study in this area is needed.
Immune therapies for TP53 myeloid disease
Immunological responses have been seen post immunosuppression withdrawal and with the utilization of donor-lymphocyte infusion.30 Studies have shown that the onset of GVHD, with its associated graft-versus-leukemia effect, can lead to improved survival.25 These findings suggest that posttransplant T-cell– directed immunological approaches may benefit these patients with high-risk disease and that rapid immune suppression taper is warranted in such scenarios.
Treatment with interferon alpha (IFN-α) can enhance dendritic cell and T-cell function and potentially the graft-versus-leukemia effect. A phase 1/2 clinical trial used pegylated IFN-α in patients undergoing HCT for high-risk or treatment-resistant AML. Patients were administered pegylated IFN-α every 14 days, beginning at day −1 before HCT, for 4 doses. At 6 months, the disease relapse rate was reduced by over 20% (39%) without an increase in post HCT toxicity and mortality.31 A trial to investigate the efficacy of IFN-α in reducing relapse in patients with TP53 mutation who are MRD negative 2 months after HCT is ongoing (NCT06130579).
Other immunological treatments, such as bispecific T-cell engagers, dual-affinity receptor-targeted antibodies, engineered T cells, CAR T cells, and CAR natural killer cells are being brought forth in AML. More study is needed to determine if these agents will have any impact in AML and particularly TP53- mutated disease.
Conclusion
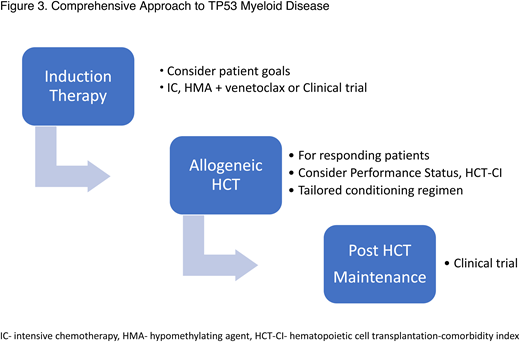
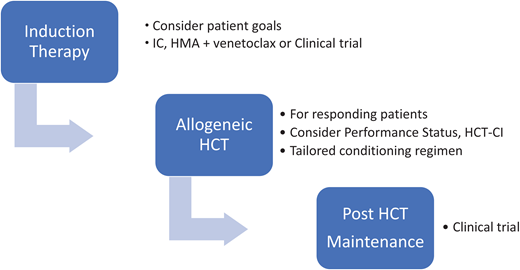
The challenge at hand is to look beyond past failures in this complex disorder. Allogeneic HCT should not be offered to all patients with TP53 myeloid disease, but a comprehensive approach should be considered for the responding, fit, and interested patients (Figure 3). Future clinical trials will hopefully improve responses in up-front treatment of the disease, broadening the patient pool eligible for HCT. Intensifying the conditioning regimen for the appropriate patients will improve outcomes. Finding the right drug or immunological approach to use in the post-transplant phase could reduce relapses and finally increase the OS in this disease.
Comprehensive approach to TP53 myeloid disease. HCT-CI, HCT-comorbidity index.
Conflict-of-interest disclosure
Hugo F. Fernández: no competing financial interests to declare.
Asmita Mishra: no competing financial interests to declare.
Off-label drug use
Hugo F. Fernández: No off-label drug discussion.
Asmita Mishra: No off-label drug discussion.